SARS-CoV-2 vaccines: a triumph of science and collaboration
- PMID: 33822773
- PMCID: PMC8262277
- DOI: 10.1172/jci.insight.149187
SARS-CoV-2 vaccines: a triumph of science and collaboration
Abstract
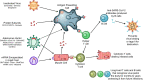
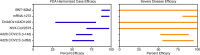
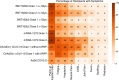
Roughly 1 year after the first case of COVID-19 was identified and less than 1 year after the sequencing of SARS-CoV-2, multiple SARS-CoV-2 vaccines with demonstrated safety and efficacy in phase III clinical trials are available. The most promising vaccines have _targeted the surface glycoprotein (S-protein) of SARS-CoV-2 and achieved an approximate 85%-95% reduction in the risk of symptomatic COVID-19, while retaining excellent safety profiles and modest side effects in the phase III clinical trials. The mRNA, replication-incompetent viral vector, and protein subunit vaccine technologies have all been successfully employed. Some novel SARS-CoV-2 variants evade but do not appear to fully overcome the potent immunity induced by these vaccines. Emerging real-world effectiveness data add evidence for protection from severe COVID-19. This is an impressive first demonstration of the effectiveness of the mRNA vaccine and vector vaccine platforms. The success of SARS-CoV-2 vaccine development should be credited to open science, industry partnerships, harmonization of clinical trials, and the altruism of study participants. The manufacturing and distribution of the emergency use-authorized SARS-CoV-2 vaccines are ongoing challenges. What remains now is to ensure broad and equitable global vaccination against COVID-19.
Conflict of interest statement
Figures
Similar articles
-
SARS-CoV-2 vaccines: A critical perspective through efficacy data and barriers to herd immunity.Respir Med. 2021 Apr-May;180:106355. doi: 10.1016/j.rmed.2021.106355. Epub 2021 Mar 6. Respir Med. 2021. PMID: 33721697 Free PMC article. Review.
-
Immune Evasive Effects of SARS-CoV-2 Variants to COVID-19 Emergency Used Vaccines.Front Immunol. 2021 Nov 22;12:771242. doi: 10.3389/fimmu.2021.771242. eCollection 2021. Front Immunol. 2021. PMID: 34880867 Free PMC article. Review.
-
Frontrunners in the race to develop a SARS-CoV-2 vaccine.Can J Microbiol. 2021 Mar;67(3):189-212. doi: 10.1139/cjm-2020-0465. Epub 2020 Dec 2. Can J Microbiol. 2021. PMID: 33264067 Review.
-
SARS-CoV-2 vaccines in advanced clinical trials: Where do we stand?Adv Drug Deliv Rev. 2021 May;172:314-338. doi: 10.1016/j.addr.2021.01.014. Epub 2021 Jan 20. Adv Drug Deliv Rev. 2021. PMID: 33482248 Free PMC article. Review.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
Cited by
-
Factors Related to Low COVID-19 Vaccination Rate in Pregnant and Postpartum Women with and without COVID-19.Rev Bras Ginecol Obstet. 2023 Nov;45(11):e638-e645. doi: 10.1055/s-0043-1772589. Epub 2023 Nov 29. Rev Bras Ginecol Obstet. 2023. PMID: 38029765 Free PMC article.
-
The COVID-19 pandemic: some thoughts on integrity in research and communication.Forensic Sci Res. 2021 Nov 9;6(4):310-315. doi: 10.1080/20961790.2021.1980953. eCollection 2021. Forensic Sci Res. 2021. PMID: 35111349 Free PMC article. No abstract available.
-
SARS-CoV-2 vaccination and multiple sclerosis: a large multicentric study on relapse risk after the third booster dose.J Neurol. 2024 Jan;271(1):24-31. doi: 10.1007/s00415-023-12034-0. Epub 2023 Nov 3. J Neurol. 2024. PMID: 37922069 Free PMC article.
-
Accelerating research and development of new vaccines against tuberculosis: a global roadmap.Lancet Infect Dis. 2022 Apr;22(4):e108-e120. doi: 10.1016/S1473-3099(21)00810-0. Epub 2022 Feb 28. Lancet Infect Dis. 2022. PMID: 35240041 Free PMC article. Review.
-
Age-Related Associations of Altruism with Attitudes towards COVID-19 and Vaccination: A Representative Survey in the North of Italy.Behav Sci (Basel). 2023 Feb 19;13(2):188. doi: 10.3390/bs13020188. Behav Sci (Basel). 2023. PMID: 36829417 Free PMC article.
References
-
- World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... Updated April 2, 2021. Accessed March 18, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

