AMPK activation by ASP4132 inhibits non-small cell lung cancer cell growth
- PMID: 33824293
- PMCID: PMC8024326
- DOI: 10.1038/s41419-021-03655-2
AMPK activation by ASP4132 inhibits non-small cell lung cancer cell growth
Abstract
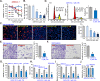
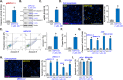
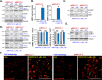
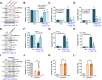
Activation of adenosine monophosphate-activated protein kinase (AMPK) is able to produce significant anti-non-small cell lung cancer (NSCLC) cell activity. ASP4132 is an orally active and highly effective AMPK activator. The current study tested its activity against NSCLC cells. In primary NSCLC cells and established cell lines (A549 and NCI-H1944) ASP4132 potently inhibited cell growth, proliferation and cell cycle progression as well as cell migration and invasion. Robust apoptosis activation was detected in ASP4132-treated NSCLC cells. Furthermore, ASP4132 treatment in NSCLC cells induced programmed necrosis, causing mitochondrial p53-cyclophilin D (CyPD)-adenine nucleotide translocase 1 (ANT1) association, mitochondrial depolarization and medium lactate dehydrogenase release. In NSCLC cells ASP4132 activated AMPK signaling, induced AMPKα1-ACC phosphorylation and increased AMPK activity. Furthermore, AMPK downstream events, including mTORC1 inhibition, receptor tyrosine kinases (PDGFRα and EGFR) degradation, Akt inhibition and autophagy induction, were detected in ASP4132-treated NSCLC cells. Importantly, AMPK inactivation by AMPKα1 shRNA, knockout (using CRISPR/Cas9 strategy) or dominant negative mutation (T172A) almost reversed ASP4132-induced anti-NSCLC cell activity. Conversely, a constitutively active AMPKα1 (T172D) mimicked and abolished ASP4132-induced actions in NSCLC cells. In vivo, oral administration of a single dose of ASP4132 largely inhibited NSCLC xenograft growth in SCID mice. AMPK activation, mTORC1 inhibition and EGFR-PDGFRα degradation as well as Akt inhibition and autophagy induction were detected in ASP4132-treated NSCLC xenograft tumor tissues. Together, activation of AMPK by ASP4132 potently inhibits NSCLC cell growth in vitro and in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Itraconazole-Induced Inhibition on Human Esophageal Cancer Cell Growth Requires AMPK Activation.Mol Cancer Ther. 2018 Jun;17(6):1229-1239. doi: 10.1158/1535-7163.MCT-17-1094. Epub 2018 Mar 28. Mol Cancer Ther. 2018. PMID: 29592879
-
Baicalin, a Chinese Herbal Medicine, Inhibits the Proliferation and Migration of Human Non-Small Cell Lung Carcinoma (NSCLC) Cells, A549 and H1299, by Activating the SIRT1/AMPK Signaling Pathway.Med Sci Monit. 2018 Apr 10;24:2126-2133. doi: 10.12659/msm.909627. Med Sci Monit. 2018. PMID: 29632297 Free PMC article.
-
Nootkatone, an AMPK activator derived from grapefruit, inhibits KRAS downstream pathway and sensitizes non-small-cell lung cancer A549 cells to adriamycin.Phytomedicine. 2019 Oct;63:153000. doi: 10.1016/j.phymed.2019.153000. Epub 2019 Jun 27. Phytomedicine. 2019. PMID: 31280139
-
_targeting AMPK by Statins: A Potential Therapeutic Approach.Drugs. 2021 Jun;81(8):923-933. doi: 10.1007/s40265-021-01510-4. Epub 2021 May 3. Drugs. 2021. PMID: 33939118 Free PMC article. Review.
-
Preclinical evidence using synthetic compounds and natural products indicates that AMPK represents a potential pharmacological _target for the therapy of pulmonary diseases.Med Res Rev. 2024 May;44(3):1326-1369. doi: 10.1002/med.22014. Epub 2024 Jan 16. Med Res Rev. 2024. PMID: 38229486 Review.
Cited by
-
Involvement of AMPKα and MAPK-ERK/-JNK Signals in Docetaxel-Induced Human Tongue Squamous Cell Carcinoma Cell Apoptosis.Int J Mol Sci. 2022 Nov 10;23(22):13857. doi: 10.3390/ijms232213857. Int J Mol Sci. 2022. PMID: 36430348 Free PMC article.
-
_targeting the mitochondrial protein YME1L to inhibit osteosarcoma cell growth in vitro and in vivo.Cell Death Dis. 2024 May 20;15(5):346. doi: 10.1038/s41419-024-06722-6. Cell Death Dis. 2024. PMID: 38769124 Free PMC article.
-
A Systematic Role of Metabolomics, Metabolic Pathways, and Chemical Metabolism in Lung Cancer.Vaccines (Basel). 2023 Feb 7;11(2):381. doi: 10.3390/vaccines11020381. Vaccines (Basel). 2023. PMID: 36851259 Free PMC article. Review.
-
HBO1 induces histone acetylation and is important for non-small cell lung cancer cell growth.Int J Biol Sci. 2022 May 9;18(8):3313-3323. doi: 10.7150/ijbs.72526. eCollection 2022. Int J Biol Sci. 2022. PMID: 35637972 Free PMC article.
-
The molecular mechanism for inhibiting the growth of nasopharyngeal carcinoma cells using polymethoxyflavonoids purified from pericarp of Citrus reticulata 'Chachi' via HSCCC.Front Pharmacol. 2023 Apr 27;14:1096001. doi: 10.3389/fphar.2023.1096001. eCollection 2023. Front Pharmacol. 2023. PMID: 37180721 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

