Modeling Type 1 Diabetes Using Pluripotent Stem Cell Technology
- PMID: 33868170
- PMCID: PMC8047192
- DOI: 10.3389/fendo.2021.635662
Modeling Type 1 Diabetes Using Pluripotent Stem Cell Technology
Abstract
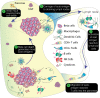
Induced pluripotent stem cell (iPSC) technology is increasingly being used to create in vitro models of monogenic human disorders. This is possible because, by and large, the phenotypic consequences of such genetic variants are often confined to a specific and known cell type, and the genetic variants themselves can be clearly identified and controlled for using a standardized genetic background. In contrast, complex conditions such as autoimmune Type 1 diabetes (T1D) have a polygenic inheritance and are subject to diverse environmental influences. Moreover, the potential cell types thought to contribute to disease progression are many and varied. Furthermore, as HLA matching is critical for cell-cell interactions in disease pathogenesis, any model that seeks to test the involvement of particular cell types must take this restriction into account. As such, creation of an in vitro model of T1D will require a system that is cognizant of genetic background and enables the interaction of cells representing multiple lineages to be examined in the context of the relevant environmental disease triggers. In addition, as many of the lineages critical to the development of T1D cannot be easily generated from iPSCs, such models will likely require combinations of cell types derived from in vitro and in vivo sources. In this review we imagine what an ideal in vitro model of T1D might look like and discuss how the required elements could be feasibly assembled using existing technologies. We also examine recent advances towards this goal and discuss potential uses of this technology in contributing to our understanding of the mechanisms underlying this autoimmune condition.
Keywords: T cell receptor; T cells; antigen presenting cells; induced pluripotent stem cells; macrophages; type 1 diabetes.
Copyright © 2021 Joshi, Cameron, Tiwari, Mannering, Elefanty and Stanley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model.Stem Cells Dev. 2012 Sep 20;21(14):2642-55. doi: 10.1089/scd.2011.0665. Epub 2012 Jun 1. Stem Cells Dev. 2012. PMID: 22512788 Free PMC article.
-
Stem cell-based multi-tissue platforms to model human autoimmune diabetes.Mol Metab. 2022 Dec;66:101610. doi: 10.1016/j.molmet.2022.101610. Epub 2022 Oct 6. Mol Metab. 2022. PMID: 36209784 Free PMC article. Review.
-
Cellular and molecular pathogenic mechanisms of insulin-dependent diabetes mellitus.Ann N Y Acad Sci. 2001 Apr;928:200-11. doi: 10.1111/j.1749-6632.2001.tb05650.x. Ann N Y Acad Sci. 2001. PMID: 11795511 Review.
-
The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes.J Autoimmun. 2016 Jan;66:76-88. doi: 10.1016/j.jaut.2015.08.019. Epub 2015 Sep 26. J Autoimmun. 2016. PMID: 26403950 Free PMC article. Review.
-
Role of innate immunity in triggering and tuning of autoimmune diabetes.Curr Mol Med. 2009 Feb;9(1):30-44. doi: 10.2174/156652409787314471. Curr Mol Med. 2009. PMID: 19199940 Review.
Cited by
-
Human Pluripotent Stem Cells Go Diabetic: A Glimpse on Monogenic Variants.Front Endocrinol (Lausanne). 2021 May 17;12:648284. doi: 10.3389/fendo.2021.648284. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34079523 Free PMC article. Review.
-
Modeling human T1D-associated autoimmune processes.Mol Metab. 2022 Feb;56:101417. doi: 10.1016/j.molmet.2021.101417. Epub 2021 Dec 10. Mol Metab. 2022. PMID: 34902607 Free PMC article. Review.
-
Autoimmune CD8+ T cells in type 1 diabetes: from single-cell RNA sequencing to T-cell receptor redirection.Front Endocrinol (Lausanne). 2024 May 10;15:1377322. doi: 10.3389/fendo.2024.1377322. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38800484 Free PMC article. Review.
-
Untangling the genetics of beta cell dysfunction and death in type 1 diabetes.Mol Metab. 2024 Aug;86:101973. doi: 10.1016/j.molmet.2024.101973. Epub 2024 Jun 22. Mol Metab. 2024. PMID: 38914291 Free PMC article. Review.
-
Towards a better understanding of diabetes mellitus using organoid models.Nat Rev Endocrinol. 2023 Apr;19(4):232-248. doi: 10.1038/s41574-022-00797-x. Epub 2023 Jan 20. Nat Rev Endocrinol. 2023. PMID: 36670309 Free PMC article. Review.
References
-
- Federation ID. International Diabetes Federation. IDF Diabetes Atlas, 9th edn.: Brussels, Belgium: International Diabetes Federation. (2019) 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

