Dahuang Zhechong Pills Suppress Silicosis Fibrosis Progression via p38 MAPK/TGF- β 1/Smad Pathway In Vitro
- PMID: 33868442
- PMCID: PMC8034999
- DOI: 10.1155/2021/6662261
Dahuang Zhechong Pills Suppress Silicosis Fibrosis Progression via p38 MAPK/TGF- β 1/Smad Pathway In Vitro
Abstract
Background: Dahuang Zhechong pills (DHZCP) is a classic Chinese medicinal prescription in "Treatise on Cold Pathogenic and Miscellaneous Diseases (Shanghan Zabing Lun)," and it has the function of tonifying blood, nourishing Yin, and removing blood stasis. Previous studies have shown that DHZCP could alleviate SiO2 induced pulmonary fibrosis in mice. This study aims to further explore the preventive and therapeutic effects of DHZCP against silicosis fibrosis and the underlying mechanisms in vitro.
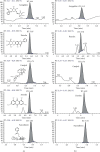
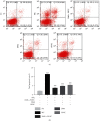
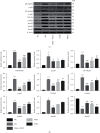
Methods: We used the experimental model of SiO2-induced MH-S cells to evaluate the therapeutic potential of DHZCP. MH-S cells induced by SiO2 were intervened with the drug-containing serum of DHZCP, and the effects of drug-containing serum of DHZCP on the MH-S cells were detected by CCK8, ELISA, flow cytometry, western blot, and immunofluorescence.
Results: DHZCP improved cell viability by reducing apoptosis. It also decreased the levels of TNF-α, IL-1β, IL-6 in the supernatant of MH-S cells induced by SiO2, inhibited the expression of p38 MAPK, blocked the activation of NF-κB, and controlled the upstream inflammatory response by multiple _targeting. Concomitantly, we observed upregulation of Smad7 and a marked decline in TGF-β1, α-SMA, Smad2, Smad3 expression in MH-S cells treated with DHZCP.
Conclusion: To sum up, we conclude that DHZCP protects against SiO2-induced silicosis by reducing the persistent irritation of inflammation, regulating the p38 MAPK/TGF-β1/Smad pathway.
Copyright © 2021 Li-Juan Wu et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Dahuang Zhechong Pill Alleviates Liver Fibrosis Progression by Regulating p38 MAPK/NF-κ B/TGF-β1 Pathway.Chin J Integr Med. 2024 Dec;30(12):1113-1120. doi: 10.1007/s11655-024-3801-x. Epub 2024 Jun 18. Chin J Integr Med. 2024. PMID: 38888716
-
Dahuang Zhechong pill attenuates hepatic sinusoidal capillarization in liver cirrhosis and hepatocellular carcinoma rat model via the MK/integrin signaling pathway.J Ethnopharmacol. 2023 May 23;308:116191. doi: 10.1016/j.jep.2023.116191. Epub 2023 Jan 31. J Ethnopharmacol. 2023. PMID: 36731809
-
Multi-material basis and multi-mechanisms of the Dahuang Zhechong pill for regulating Treg/Th1 balance in hepatocellular carcinoma.Phytomedicine. 2022 Jun;100:154055. doi: 10.1016/j.phymed.2022.154055. Epub 2022 Mar 15. Phytomedicine. 2022. PMID: 35344716
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential _targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
-
Diverse roles of TGF-β/Smads in renal fibrosis and inflammation.Int J Biol Sci. 2011;7(7):1056-67. doi: 10.7150/ijbs.7.1056. Epub 2011 Sep 2. Int J Biol Sci. 2011. PMID: 21927575 Free PMC article. Review.
Cited by
-
From Basic Research to Clinical Practice: Considerations for Treatment Drugs for Silicosis.Int J Mol Sci. 2023 May 5;24(9):8333. doi: 10.3390/ijms24098333. Int J Mol Sci. 2023. PMID: 37176040 Free PMC article. Review.
-
A Novel N-Arylpyridone Compound Alleviates the Inflammatory and Fibrotic Reaction of Silicosis by Inhibiting the ASK1-p38 Pathway and Regulating Macrophage Polarization.Front Pharmacol. 2022 Mar 23;13:848435. doi: 10.3389/fphar.2022.848435. eCollection 2022. Front Pharmacol. 2022. PMID: 35401236 Free PMC article.
-
Yangqing Chenfei formula alleviates crystalline silica induced pulmonary inflammation and fibrosis by suppressing macrophage polarization.J Tradit Chin Med. 2023 Oct;43(6):1126-1139. doi: 10.19852/j.cnki.jtcm.20230517.003. J Tradit Chin Med. 2023. PMID: 37946475 Free PMC article.
-
Standardized Edible Bird's Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin.Antioxidants (Basel). 2021 Sep 13;10(9):1452. doi: 10.3390/antiox10091452. Antioxidants (Basel). 2021. PMID: 34573084 Free PMC article.
-
Dahuang Zhechong Pill Alleviates Liver Fibrosis Progression by Regulating p38 MAPK/NF-κ B/TGF-β1 Pathway.Chin J Integr Med. 2024 Dec;30(12):1113-1120. doi: 10.1007/s11655-024-3801-x. Epub 2024 Jun 18. Chin J Integr Med. 2024. PMID: 38888716
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

