Synthesis, biological evaluation and docking studies of 1,2,4-oxadiazole linked 5-fluorouracil derivatives as anticancer agents
- PMID: 33947440
- PMCID: PMC8097950
- DOI: 10.1186/s13065-021-00757-y
Synthesis, biological evaluation and docking studies of 1,2,4-oxadiazole linked 5-fluorouracil derivatives as anticancer agents
Abstract
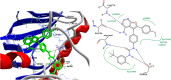
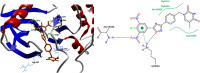
Background: 1,2,4-oxadiazole derivatives exhibited significant anti-cancer activity when they were evaluated, against human cancer cell lines. They also showed anti-inflammatory, analgesic, diabetic, immunosuppressive, α,β3-receptor antagonist, antimicrobial, anti-helminthic, histamine-H3 and antiparasitic properties. A pyrimidine analog, 5 fluoro-uracil is a chemotherapeutic drug used for treating multiple solid malignant tumors. But its application is limited, as it has side effects like low bioavailability and high toxicity. Molecular docking is an exemplary tool, helps in identifying _target and designing a drug containing high bio-availability and minimum toxicity.
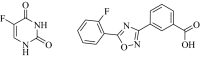
Results: A set of 1,2,4-oxadiazole linked 5-fluoruracil derivatives (7a-j) were synthesized and their structures were confirmed by 1HNMR, 13CNMR and Mass spectral analysis. Further, these compounds were investigated for their anticancer activity towards a panel of four human cancer cell lines such as (MCF-7, MDA MB-231), lung cancer (A549) and prostate cancer (DU-145) by using MTT method. Among them, compounds 7a, 7b, 7c, 7d and 7i demonstrated more promising anticancer activity than standard.
Conclusion: Synthesized derivatives (7a-j) of 1,2,4-oxadiazole linked 5-fluorouracil and investigated for their anticancer activity towards a panel of four human cancer cell lines.
Keywords: 5-Fluorouracil; Ataluren; Oxadiazole and anticancer activity; Pyrimidine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
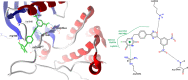
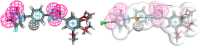
Similar articles
-
Investigations of p-tolyloxy-1,3,4-oxadiazole propionamides as soybean 15-lipoxygenase inhibitors in comforting with in vitro and in silico studies.J Biomol Struct Dyn. 2023;41(24):15549-15568. doi: 10.1080/07391102.2023.2190807. Epub 2023 Mar 22. J Biomol Struct Dyn. 2023. PMID: 36946200
-
Design, synthesis, anticancer evaluation and molecular docking studies of 1,2,3-triazole incorporated 1,3,4-oxadiazole-Triazine derivatives.Heliyon. 2023 May 3;9(5):e15935. doi: 10.1016/j.heliyon.2023.e15935. eCollection 2023 May. Heliyon. 2023. PMID: 37206039 Free PMC article.
-
Computational and Molecular Docking Studies of New Benzene Sulfonamide Drugs with Anticancer and Antioxidant Effects.Curr Org Synth. 2023;20(3):339-350. doi: 10.2174/1570179420666221007141937. Curr Org Synth. 2023. PMID: 36214306
-
Design, Synthesis and Cytotoxicity Evalufation of Substituted Benzimidazole Conjugated 1,3,4-Oxadiazoles.Chem Pharm Bull (Tokyo). 2022;70(6):448-453. doi: 10.1248/cpb.c22-00162. Chem Pharm Bull (Tokyo). 2022. PMID: 35650042
-
Exploring Therapeutic Potential of 1,3,4-Oxadiazole Nucleus as Anticancer Agents: A Mini-review.Med Chem. 2023;19(2):119-131. doi: 10.2174/1573406418666220608120908. Med Chem. 2023. PMID: 35676848 Review.
Cited by
-
Synthesis, molecular docking study and anticancer activity of novel 1,3,4-oxadiazole derivatives as potential tubulin inhibitors.Heliyon. 2023 Feb 8;9(2):e13460. doi: 10.1016/j.heliyon.2023.e13460. eCollection 2023 Feb. Heliyon. 2023. PMID: 36846693 Free PMC article.
-
Enzyme kinetics of deoxyuridine triphosphatase from Western corn rootworm.BMC Res Notes. 2023 Nov 16;16(1):336. doi: 10.1186/s13104-023-06618-2. BMC Res Notes. 2023. PMID: 37974243 Free PMC article.
-
Synthesis, Biological Evaluation, DNA Binding, and Molecular Docking of Hybrid 4,6-Dihydrazone Pyrimidine Derivatives as Antitumor Agents.Molecules. 2022 Dec 26;28(1):187. doi: 10.3390/molecules28010187. Molecules. 2022. PMID: 36615380 Free PMC article.
References
-
- Ghomi JS, Ghasemzadeh MA. An efficient route to the synthesis of pyrimidine-2-ones under ultrasound irradiation. Dig J Nanomater Biostruct. 2010;5:303–306.
-
- Ukrainets IV, Tugaibei IA, Bereznykova NL, Karvechenko VN, Turov AV. 4-Hydroxy-2-quinolones 144. Alkyl-, arylalkyl- and arylamides of 2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxylic acid and their diuretic properties. Chem Heterocycl Compd. 2008;5:565–575. doi: 10.1007/s10593-008-0076-7. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
