Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data
- PMID: 33964222
- PMCID: PMC8099315
- DOI: 10.1016/S0140-6736(21)00947-8
Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data
Erratum in
-
Department of Error.Lancet. 2021 Jul 17;398(10296):212. doi: 10.1016/S0140-6736(21)01555-5. Lancet. 2021. PMID: 34274063 Free PMC article. No abstract available.
Abstract
Background: Following the emergency use authorisation of the Pfizer-BioNTech mRNA COVID-19 vaccine BNT162b2 (international non-proprietary name tozinameran) in Israel, the Ministry of Health (MoH) launched a campaign to immunise the 6·5 million residents of Israel aged 16 years and older. We estimated the real-world effectiveness of two doses of BNT162b2 against a range of SARS-CoV-2 outcomes and to evaluate the nationwide public-health impact following the widespread introduction of the vaccine.
Methods: We used national surveillance data from the first 4 months of the nationwide vaccination campaign to ascertain incident cases of laboratory-confirmed SARS-CoV-2 infections and outcomes, as well as vaccine uptake in residents of Israel aged 16 years and older. Vaccine effectiveness against SARS-CoV-2 outcomes (asymptomatic infection, symptomatic infection, and COVID-19-related hospitalisation, severe or critical hospitalisation, and death) was calculated on the basis of incidence rates in fully vaccinated individuals (defined as those for whom 7 days had passed since receiving the second dose of vaccine) compared with rates in unvaccinated individuals (who had not received any doses of the vaccine), with use of a negative binomial regression model adjusted for age group (16-24, 25-34, 35-44, 45-54, 55-64, 65-74, 75-84, and ≥85 years), sex, and calendar week. The proportion of spike gene _target failures on PCR test among a nationwide convenience-sample of SARS-CoV-2-positive specimens was used to estimate the prevelance of the B.1.1.7 variant.
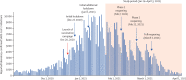
Findings: During the analysis period (Jan 24 to April 3, 2021), there were 232 268 SARS-CoV-2 infections, 7694 COVID-19 hospitalisations, 4481 severe or critical COVID-19 hospitalisations, and 1113 COVID-19 deaths in people aged 16 years or older. By April 3, 2021, 4 714 932 (72·1%) of 6 538 911 people aged 16 years and older were fully vaccinated with two doses of BNT162b2. Adjusted estimates of vaccine effectiveness at 7 days or longer after the second dose were 95·3% (95% CI 94·9-95·7; incidence rate 91·5 per 100 000 person-days in unvaccinated vs 3·1 per 100 000 person-days in fully vaccinated individuals) against SARS-CoV-2 infection, 91·5% (90·7-92·2; 40·9 vs 1·8 per 100 000 person-days) against asymptomatic SARS-CoV-2 infection, 97·0% (96·7-97·2; 32·5 vs 0·8 per 100 000 person-days) against symptomatic COVID-19, 97·2% (96·8-97·5; 4·6 vs 0·3 per 100 000 person-days) against COVID-19-related hospitalisation, 97·5% (97·1-97·8; 2·7 vs 0·2 per 100 000 person-days) against severe or critical COVID-19-related hospitalisation, and 96·7% (96·0-97·3; 0·6 vs 0·1 per 100 000 person-days) against COVID-19-related death. In all age groups, as vaccine coverage increased, the incidence of SARS-CoV-2 outcomes declined. 8006 of 8472 samples tested showed a spike gene _target failure, giving an estimated prevalence of the B.1.1.7 variant of 94·5% among SARS-CoV-2 infections.
Interpretation: Two doses of BNT162b2 are highly effective across all age groups (≥16 years, including older adults aged ≥85 years) in preventing symptomatic and asymptomatic SARS-CoV-2 infections and COVID-19-related hospitalisations, severe disease, and death, including those caused by the B.1.1.7 SARS-CoV-2 variant. There were marked and sustained declines in SARS-CoV-2 incidence corresponding to increasing vaccine coverage. These findings suggest that COVID-19 vaccination can help to control the pandemic.
Funding: None.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests FJA, JMM, FK, GM, KP, JS, DLS, and LJ hold stock and stock options in Pfizer. All other authors declare no competing interests.
Figures

Comment in
-
COVID-19 vaccine impact in Israel and a way out of the pandemic.Lancet. 2021 May 15;397(10287):1783-1785. doi: 10.1016/S0140-6736(21)01018-7. Epub 2021 May 5. Lancet. 2021. PMID: 33964221 Free PMC article. No abstract available.
Similar articles
-
Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study.Lancet Infect Dis. 2022 Mar;22(3):357-366. doi: 10.1016/S1473-3099(21)00566-1. Epub 2021 Sep 22. Lancet Infect Dis. 2022. PMID: 34562375 Free PMC article.
-
Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers.JAMA. 2021 Jun 22;325(24):2457-2465. doi: 10.1001/jama.2021.7152. JAMA. 2021. PMID: 33956048 Free PMC article.
-
Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study.Lancet Infect Dis. 2021 Nov;21(11):1529-1538. doi: 10.1016/S1473-3099(21)00289-9. Epub 2021 Jun 23. Lancet Infect Dis. 2021. PMID: 34174193 Free PMC article.
-
Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis.Infect Dis Poverty. 2021 Nov 14;10(1):132. doi: 10.1186/s40249-021-00915-3. Infect Dis Poverty. 2021. PMID: 34776011 Free PMC article. Review.
-
Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review.Clin Microbiol Infect. 2022 Feb;28(2):202-221. doi: 10.1016/j.cmi.2021.10.005. Epub 2021 Oct 27. Clin Microbiol Infect. 2022. PMID: 34715347 Free PMC article. Review.
Cited by
-
Safety and immune response kinetics of GRAd-COV2 vaccine: phase 1 clinical trial results.NPJ Vaccines. 2022 Sep 24;7(1):111. doi: 10.1038/s41541-022-00531-8. NPJ Vaccines. 2022. PMID: 36153335 Free PMC article.
-
Experiencing herd immunity in virtual reality increases COVID-19 vaccination intention: Evidence from a large-scale field intervention study.Comput Human Behav. 2023 Feb;139:107533. doi: 10.1016/j.chb.2022.107533. Epub 2022 Oct 17. Comput Human Behav. 2023. PMID: 36277032 Free PMC article.
-
A Bivalent Omicron-BA.4/BA.5-Adapted BNT162b2 Booster in ≥12-Year-Olds.Clin Infect Dis. 2024 May 15;78(5):1194-1203. doi: 10.1093/cid/ciad718. Clin Infect Dis. 2024. PMID: 38016021 Free PMC article. Clinical Trial.
-
Clinical features and 28-day mortality predictors of vaccinated patients admitted to a COVID-19 ICU hub in Italy.J Anesth Analg Crit Care. 2023 Nov 13;3(1):47. doi: 10.1186/s44158-023-00130-6. J Anesth Analg Crit Care. 2023. PMID: 37957713 Free PMC article.
-
Semliki Forest Virus (SFV) Self-Amplifying RNA Delivered to J774A.1 Macrophage Lineage by Its Association with a Purified Recombinant SFV Capsid Protein.Int J Mol Sci. 2024 Jul 18;25(14):7859. doi: 10.3390/ijms25147859. Int J Mol Sci. 2024. PMID: 39063100 Free PMC article.
References
-
- Worldometer COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus
-
- Israel Ministry of Health COVID-19 database (in Hebrew) https://data.gov.il/dataset/covid-19
-
- Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. - PubMed
-
- Ayyub R. Reuters; Dec 23, 2020. UK COVID-19 variant detected in Israel, health ministry says.https://www.reuters.com/article/uk-health-coronavirus-israel/uk-covid-19...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

