Regulation and distinct physiological roles of manganese in bacteria
- PMID: 34037759
- PMCID: PMC8632737
- DOI: 10.1093/femsre/fuab028
Regulation and distinct physiological roles of manganese in bacteria
Abstract
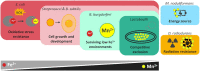
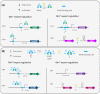
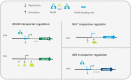
Manganese (Mn2+) is an essential trace element within organisms spanning the entire tree of life. In this review, we provide an overview of Mn2+ transport and the regulation of its homeostasis in bacteria, with a focus on its functions beyond being a cofactor for enzymes. Crucial differences in Mn2+ homeostasis exist between bacterial species that can be characterized to have an iron- or manganese-centric metabolism. Highly iron-centric species require minimal Mn2+ and mostly use it as a mechanism to cope with oxidative stress. As a consequence, tight regulation of Mn2+ uptake is required, while organisms that use both Fe2+ and Mn2+ need other layers of regulation for maintaining homeostasis. We will focus in detail on manganese-centric bacterial species, in particular lactobacilli, that require little to no Fe2+ and use Mn2+ for a wider variety of functions. These organisms can accumulate extraordinarily high amounts of Mn2+ intracellularly, enabling the nonenzymatic use of Mn2+ for decomposition of reactive oxygen species while simultaneously functioning as a mechanism of competitive exclusion. We further discuss how Mn2+ accumulation can provide both beneficial and pathogenic bacteria with advantages in thriving in their niches.
Keywords: bacilli; competitive exclusion; lactobacilli; manganese; oxidative stress.
© The Author(s) 2021. Published by Oxford University Press on behalf of FEMS.
Figures


Similar articles
-
Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria.FEMS Microbiol Rev. 2003 Jun;27(2-3):263-90. doi: 10.1016/S0168-6445(03)00052-4. FEMS Microbiol Rev. 2003. PMID: 12829271 Review.
-
Mn2+ and bacterial pathogenesis.Front Biosci. 2004 May 1;9:1035-42. doi: 10.2741/1317. Front Biosci. 2004. PMID: 14977526 Review.
-
The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen.Mol Microbiol. 2000 Jun;36(5):1085-100. doi: 10.1046/j.1365-2958.2000.01922.x. Mol Microbiol. 2000. PMID: 10844693
-
Competitive Exclusion Is a Major Bioprotective Mechanism of Lactobacilli against Fungal Spoilage in Fermented Milk Products.Appl Environ Microbiol. 2020 Mar 18;86(7):e02312-19. doi: 10.1128/AEM.02312-19. Print 2020 Mar 18. Appl Environ Microbiol. 2020. PMID: 32005739 Free PMC article.
-
[Study on separation of bacteria on the surface of mature manganese sand and the ability of oxidating Fe2+ and Mn2+].Huan Jing Ke Xue. 2011 Jan;32(1):125-9. Huan Jing Ke Xue. 2011. PMID: 21404675 Chinese.
Cited by
-
Bioprotection Potential of Lacticaseibacillus rhamnosus LRH01 and Lactiplantibacillus plantarum LP01 against Spoilage-Associated Penicillium Strains in Yoghurt.Molecules. 2023 Nov 2;28(21):7397. doi: 10.3390/molecules28217397. Molecules. 2023. PMID: 37959814 Free PMC article.
-
SloR-SRE binding to the S. mutans mntH promoter is cooperative.bioRxiv [Preprint]. 2024 Nov 3:2024.11.02.621577. doi: 10.1101/2024.11.02.621577. bioRxiv. 2024. PMID: 39554117 Free PMC article. Preprint.
-
ZccE is a Novel P-type ATPase That Protects Streptococcus mutans Against Zinc Intoxication.PLoS Pathog. 2022 Aug 8;18(8):e1010477. doi: 10.1371/journal.ppat.1010477. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35939512 Free PMC article.
-
Antimicrobial blue light inactivation of Pseudomonas aeruginosa: Unraveling the multifaceted impact of wavelength, growth stage, and medium composition.J Photochem Photobiol B. 2024 Oct;259:113023. doi: 10.1016/j.jphotobiol.2024.113023. Epub 2024 Aug 30. J Photochem Photobiol B. 2024. PMID: 39241393
-
Manganese Transporter Proteins in Salmonella enterica serovar Typhimurium.J Microbiol. 2023 Mar;61(3):289-296. doi: 10.1007/s12275-023-00027-7. Epub 2023 Mar 2. J Microbiol. 2023. PMID: 36862278 Review.
References
-
- Archibald F. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol Lett. 1983;19:29–32.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

