The Ferroxidase Hephaestin in Lung Cancer: Pathological Significance and Prognostic Value
- PMID: 34094919
- PMCID: PMC8170403
- DOI: 10.3389/fonc.2021.638856
The Ferroxidase Hephaestin in Lung Cancer: Pathological Significance and Prognostic Value
Abstract
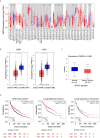
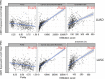
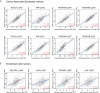
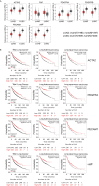
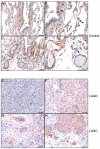
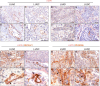
Hephaestin (HEPH) belongs to a group of exocytoplasmic ferroxidases which contribute to cellular iron homeostasis by favouring its export. Down-regulation of HEPH expression, possibly by stimulating cell proliferation due to an increase in iron availability, has shown to correlate with poor survival in breast cancer. The lung is particularly sensitive to iron-induced oxidative stress, given the high oxygen tension present, however, HEPH distribution in lung cancer and its influence on prognosis have not been investigated yet. In this study we explored the prognostic value of HEPH and its expression pattern in the most prevalent histotypes of lung cancers, namely lung adenocarcinoma and lung squamous cell carcinoma. In silico analyses, based on UALCAN, Gene Expression Profiling Interactive Analysis (GEPIA) and Kaplan-Meier plotter bioinformatics, revealed a significant correlation between higher levels of HEPH expression and favorable prognosis, in both cancer histotypes. Moreover, TIMER web platform showed a statistically significant association between HEPH expression and cell elements belonging to the tumor microenvironment identified as endothelial cells and a subpopulation of cancer-associated fibroblasts, further confirmed by double immunohistochemical labeling with cell type specific markers. Taken together, these data shed a light on the complex mechanisms of local iron handling lung cancer can exploit to support tumorigenesis.
Keywords: bioinformatics; hephaestin; immunohistochemistry; iron; lung cancer.
Copyright © 2021 Zacchi, Belmonte, Mangogna, Morello, Scola, Martorana and Borelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The biology of mammalian multi-copper ferroxidases.Biometals. 2023 Apr;36(2):263-281. doi: 10.1007/s10534-022-00370-z. Epub 2022 Feb 15. Biometals. 2023. PMID: 35167013 Free PMC article. Review.
-
Severe Iron Metabolism Defects in Mice With Double Knockout of the Multicopper Ferroxidases Hephaestin and Ceruloplasmin.Cell Mol Gastroenterol Hepatol. 2018 Jun 23;6(4):405-427. doi: 10.1016/j.jcmgh.2018.06.006. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30182051 Free PMC article.
-
CDX2-regulated expression of iron transport protein hephaestin in intestinal and colonic epithelium.Gastroenterology. 2005 Apr;128(4):946-61. doi: 10.1053/j.gastro.2005.01.003. Gastroenterology. 2005. PMID: 15825077
-
Deletion of hephaestin and ceruloplasmin induces a serious systemic iron deficiency and disrupts iron homeostasis.Biochem Biophys Res Commun. 2018 Sep 10;503(3):1905-1910. doi: 10.1016/j.bbrc.2018.07.134. Epub 2018 Jul 27. Biochem Biophys Res Commun. 2018. PMID: 30060949
-
Hephaestin--a ferroxidase of cellular iron export.Int J Biochem Cell Biol. 2005 Jun;37(6):1173-8. doi: 10.1016/j.biocel.2004.12.007. Epub 2005 Jan 26. Int J Biochem Cell Biol. 2005. PMID: 15778082 Review.
Cited by
-
The biology of mammalian multi-copper ferroxidases.Biometals. 2023 Apr;36(2):263-281. doi: 10.1007/s10534-022-00370-z. Epub 2022 Feb 15. Biometals. 2023. PMID: 35167013 Free PMC article. Review.
-
A genomic perspective of the aging human and mouse lung with a focus on immune response and cellular senescence.Immun Ageing. 2023 Nov 6;20(1):58. doi: 10.1186/s12979-023-00373-5. Immun Ageing. 2023. PMID: 37932771 Free PMC article.
-
Iron chelators as a therapeutic option for Alzheimer's disease-A mini-review.Front Aging. 2023 Aug 2;4:1234958. doi: 10.3389/fragi.2023.1234958. eCollection 2023. Front Aging. 2023. PMID: 37602277 Free PMC article. Review.
-
A Bioinformatics-Assisted Review on Iron Metabolism and Immune System to Identify Potential Biomarkers of Exercise Stress-Induced Immunosuppression.Biomedicines. 2022 Mar 21;10(3):724. doi: 10.3390/biomedicines10030724. Biomedicines. 2022. PMID: 35327526 Free PMC article. Review.
-
UBE2S promotes the development of ovarian cancer by promoting PI3K/AKT/mTOR signaling pathway to regulate cell cycle and apoptosis.Mol Med. 2022 Jun 3;28(1):62. doi: 10.1186/s10020-022-00489-2. Mol Med. 2022. PMID: 35658829 Free PMC article.
References
LinkOut - more resources
Full Text Sources

