The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment
- PMID: 34095111
- PMCID: PMC8173135
- DOI: 10.3389/fcell.2021.637675
The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment
Abstract
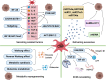
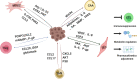
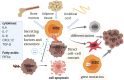
Cancer cells resistance to various therapies remains to be a key challenge nowadays. For a long time, scientists focused on tumor cells themselves for the mechanisms of acquired drug resistance. However, recent evidence showed that tumor microenvironment (TME) is essential for regulating immune escape, drug resistance, progression and metastasis of malignant cells. Reciprocal interactions between cancer cells and non-malignant cells within this milieu often reshape the TME and promote drug resistance. Therefore, advanced knowledge about these sophisticated interactions is significant for the design of effective therapeutic approaches. In this review, we highlight cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs), T-regulatory lymphocytes (Tregs), mesenchymal stem cells (MSCs), cancer-associated adipocytes (CAAs), and tumor endothelial cells (TECs) existing in TME, as well as their multiple cross-talk with tumor cells, which eventually endows tumor cells with therapeutic resistance.
Keywords: MSCs; CAFs; TAMs; antineoplastic drug resistance; cell-cell interplays; tumor microenvironment.
Copyright © 2021 Ni, Zhou, Yang, Shi, Li, Zhao and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment.World J Stem Cells. 2015 Mar 26;7(2):253-65. doi: 10.4252/wjsc.v7.i2.253. World J Stem Cells. 2015. PMID: 25815113 Free PMC article. Review.
-
Tumor-associated Macrophages (TAMs) in Cancer Resistance; Modulation by Natural Products.Curr Top Med Chem. 2023;23(12):1104-1122. doi: 10.2174/1568026623666230201145909. Curr Top Med Chem. 2023. PMID: 36722486 Review.
-
Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment.Adv Pharm Bull. 2020 Sep;10(4):556-565. doi: 10.34172/apb.2020.066. Epub 2020 Aug 9. Adv Pharm Bull. 2020. PMID: 33062602 Free PMC article. Review.
-
_targeting of the tumor immune microenvironment by metformin.J Cell Commun Signal. 2022 Sep;16(3):333-348. doi: 10.1007/s12079-021-00648-w. Epub 2021 Oct 5. J Cell Commun Signal. 2022. PMID: 34611852 Free PMC article. Review.
-
Modulation of the tumor microenvironment (TME) by melatonin.Eur J Pharmacol. 2021 Sep 15;907:174365. doi: 10.1016/j.ejphar.2021.174365. Epub 2021 Jul 21. Eur J Pharmacol. 2021. PMID: 34302814 Review.
Cited by
-
The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance.Biology (Basel). 2024 Feb 19;13(2):130. doi: 10.3390/biology13020130. Biology (Basel). 2024. PMID: 38392348 Free PMC article. Review.
-
The anti-neoplastic effects of metformin modulate the acquired phenotype of fibroblast cells in the breast cancer-normal fibroblast co-culture system.Oncol Res. 2024 Feb 6;32(3):477-487. doi: 10.32604/or.2023.043926. eCollection 2024. Oncol Res. 2024. PMID: 38361760 Free PMC article.
-
The role of tumor microenvironment in drug resistance: emerging technologies to unravel breast cancer heterogeneity.Front Oncol. 2023 May 17;13:1170264. doi: 10.3389/fonc.2023.1170264. eCollection 2023. Front Oncol. 2023. PMID: 37265795 Free PMC article. Review.
-
Overcoming Barriers in Photodynamic Therapy Harnessing Nanogenerators Strategies.Int J Biol Sci. 2024 Oct 14;20(14):5673-5694. doi: 10.7150/ijbs.100317. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494340 Free PMC article. Review.
-
Combinational Gene Therapy toward Cancer with Nanoplatform: Strategies and Principles.ACS Mater Au. 2023 Jul 27;3(6):584-599. doi: 10.1021/acsmaterialsau.3c00035. eCollection 2023 Nov 8. ACS Mater Au. 2023. PMID: 38089659 Free PMC article. Review.
References
-
- Aggen D. H., Ager C. R., Obradovic A. Z., Chowdhury N., Ghasemzadeh A., Mao W., et al. (2021). Blocking IL1 beta promotes tumor regression and remodeling of the myeloid compartment in a renal cell carcinoma model: multidimensional analyses. Clin. Cancer Res. 27 608–621. 10.1158/1078-0432.CCR-20-1610 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

