WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression
- PMID: 34108491
- PMCID: PMC8190270
- DOI: 10.1038/s41467-021-23864-9
WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression
Abstract
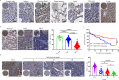
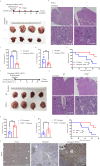
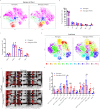
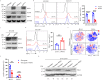
WSX1, a receptor subunit for IL-27, is widely expressed in immune cells and closely involved in immune response, but its function in nonimmune cells remains unknown. Here we report that WSX1 is highly expressed in human hepatocytes but downregulated in hepatocellular carcinoma (HCC) cells. Using NRAS/AKT-derived spontaneous HCC mouse models, we reveal an IL-27-independent tumor-suppressive effect of WSX1 that largely relies on CD8+ T-cell immune surveillance via reducing neoplastic PD-L1 expression and the associated CD8+ T-cell exhaustion. Mechanistically, WSX1 transcriptionally downregulates an isoform of PI3K-PI3Kδ and thereby inactivates AKT, reducing AKT-induced GSK3β inhibition. Activated GSK3β then boosts PD-L1 degradation, resulting in PD-L1 reduction. Overall, we demonstrate that WSX1 is a tumor suppressor that reinforces hepatic immune surveillance by blocking the PI3Kδ/AKT/GSK3β/PD-L1 pathway. Our results may yield insights into the host homeostatic control of immune response and benefit the development of cancer immunotherapies.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
HGF/c-MET pathway contributes to cisplatin-mediated PD-L1 expression in hepatocellular carcinoma.Cell Biol Int. 2021 Dec;45(12):2521-2533. doi: 10.1002/cbin.11697. Epub 2021 Sep 15. Cell Biol Int. 2021. PMID: 34486197
-
FAT10 induces immune suppression by upregulating PD-L1 expression in hepatocellular carcinoma.Apoptosis. 2024 Oct;29(9-10):1529-1545. doi: 10.1007/s10495-024-01982-1. Epub 2024 Jun 2. Apoptosis. 2024. PMID: 38824477
-
IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma.J Immunother Cancer. 2020 Jun;8(1):e000285. doi: 10.1136/jitc-2019-000285. J Immunother Cancer. 2020. PMID: 32581055 Free PMC article.
-
Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action.Expert Opin Drug Deliv. 2021 Feb;18(2):187-203. doi: 10.1080/17425247.2021.1825376. Epub 2020 Oct 5. Expert Opin Drug Deliv. 2021. PMID: 32954856 Review.
-
Progress of PD-1/PD-L1 signaling in immune response to liver transplantation for hepatocellular carcinoma.Front Immunol. 2023 Jul 20;14:1227756. doi: 10.3389/fimmu.2023.1227756. eCollection 2023. Front Immunol. 2023. PMID: 37545535 Free PMC article. Review.
Cited by
-
As a prognostic biomarker of clear cell renal cell carcinoma RUFY4 predicts immunotherapy responsiveness in a PDL1-related manner.Cancer Cell Int. 2022 Feb 8;22(1):66. doi: 10.1186/s12935-022-02480-7. Cancer Cell Int. 2022. PMID: 35135552 Free PMC article.
-
The current status and future of PD-L1 in liver cancer.Front Immunol. 2023 Dec 12;14:1323581. doi: 10.3389/fimmu.2023.1323581. eCollection 2023. Front Immunol. 2023. PMID: 38155974 Free PMC article. Review.
-
Beyond inhibition against the PD-1/PD-L1 pathway: development of PD-L1 inhibitors _targeting internalization and degradation of PD-L1.RSC Med Chem. 2023 Dec 21;15(4):1096-1108. doi: 10.1039/d3md00636k. eCollection 2024 Apr 24. RSC Med Chem. 2023. PMID: 38665824 Free PMC article. Review.
-
TRIM22 induces cellular senescence by _targeting PHLPP2 in hepatocellular carcinoma.Cell Death Dis. 2024 Jan 10;15(1):26. doi: 10.1038/s41419-024-06427-w. Cell Death Dis. 2024. PMID: 38199981 Free PMC article.
-
Transforming growth factor-β signalling in tumour resistance to the anti-PD-(L)1 therapy: Updated.J Cell Mol Med. 2023 Feb;27(3):311-321. doi: 10.1111/jcmm.17666. Epub 2023 Jan 10. J Cell Mol Med. 2023. PMID: 36625080 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

