Neurological Implications of COVID-19: Role of Redox Imbalance and Mitochondrial Dysfunction
- PMID: 34110602
- PMCID: PMC8190166
- DOI: 10.1007/s12035-021-02412-y
Neurological Implications of COVID-19: Role of Redox Imbalance and Mitochondrial Dysfunction
Abstract
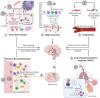
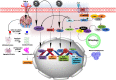
Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 or COVID-19 has been declared as a pandemic disease by the World Health Organization (WHO). Globally, this disease affected 159 million of the population and reported ~ 3.3 million deaths to the current date (May 2021). There is no definitive treatment strategy that has been identified, although this disease has prevailed in its current form for the past 18 months. The main challenges in the (SARS-CoV)-2 infections are in identifying the heterogeneity in viral strains and the plausible mechanisms of viral infection to human tissues. In parallel to the investigations into the patho-mechanism of SARS-CoV-2 infection, understanding the fundamental processes underlying the clinical manifestations of COVID-19 is very crucial for designing effective therapies. Since neurological symptoms are very apparent in COVID-19 infected patients, here, we tried to emphasize the involvement of redox imbalance and subsequent mitochondrial dysfunction in the progression of the COVID-19 infection. It has been articulated that mitochondrial dysfunction is very apparent and also interlinked to neurological symptoms in COVID-19 infection. Overall, this article provides an in-depth overview of redox imbalance and mitochondrial dysfunction involvement in aggravating COVID-19 infection and its probable contribution to the neurological manifestation of the disease.
Keywords: Bioenergetic sensors; COVID-19; Mitochondria; Neurological manifestations; Redox imbalance.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Immune landscape and redox imbalance during neurological disorders in COVID-19.Cell Death Dis. 2023 Sep 6;14(9):593. doi: 10.1038/s41419-023-06102-6. Cell Death Dis. 2023. PMID: 37673862 Free PMC article. Review.
-
Emerging COVID-19 Neurological Manifestations: Present Outlook and Potential Neurological Challenges in COVID-19 Pandemic.Mol Neurobiol. 2021 Sep;58(9):4694-4715. doi: 10.1007/s12035-021-02450-6. Epub 2021 Jun 24. Mol Neurobiol. 2021. PMID: 34169443 Free PMC article. Review.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Impact on the Central Nervous System: Are Astrocytes and Microglia Main Players or Merely Bystanders?ASN Neuro. 2020 Jan-Dec;12:1759091420954960. doi: 10.1177/1759091420954960. ASN Neuro. 2020. PMID: 32878468 Free PMC article. Review.
-
Neuroinvasion and neurotropism of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin Microbiol. 2024 Jun;79:102474. doi: 10.1016/j.mib.2024.102474. Epub 2024 Apr 13. Curr Opin Microbiol. 2024. PMID: 38615394 Review.
-
Severe acute respiratory syndrome coronavirus 2 infection reaches the human nervous system: How?J Neurosci Res. 2021 Mar;99(3):750-777. doi: 10.1002/jnr.24752. Epub 2020 Nov 20. J Neurosci Res. 2021. PMID: 33217763 Free PMC article. Review.
Cited by
-
Immune landscape and redox imbalance during neurological disorders in COVID-19.Cell Death Dis. 2023 Sep 6;14(9):593. doi: 10.1038/s41419-023-06102-6. Cell Death Dis. 2023. PMID: 37673862 Free PMC article. Review.
-
COVID-19 metabolism: Mechanisms and therapeutic _targets.MedComm (2020). 2022 Aug 9;3(3):e157. doi: 10.1002/mco2.157. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35958432 Free PMC article. Review.
-
Can SARS-CoV-2 Infection Lead to Neurodegeneration and Parkinson's Disease?Brain Sci. 2021 Dec 18;11(12):1654. doi: 10.3390/brainsci11121654. Brain Sci. 2021. PMID: 34942956 Free PMC article. Review.
-
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.NPJ Sci Food. 2024 Mar 30;8(1):19. doi: 10.1038/s41538-024-00261-2. NPJ Sci Food. 2024. PMID: 38555403 Free PMC article. Review.
-
Does COVID-19 Trigger the Risk for the Development of Parkinson's Disease? Therapeutic Potential of Vitamin C.Mol Neurobiol. 2024 Dec;61(12):9945-9960. doi: 10.1007/s12035-023-03756-3. Epub 2023 Nov 14. Mol Neurobiol. 2024. PMID: 37957424 Review.
References
-
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-05042762808. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

