Diagnostic and prognostic potential of the proteomic profiling of serum-derived extracellular vesicles in prostate cancer
- PMID: 34155195
- PMCID: PMC8215487
- DOI: 10.1038/s41419-021-03909-z
Diagnostic and prognostic potential of the proteomic profiling of serum-derived extracellular vesicles in prostate cancer
Abstract
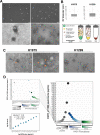
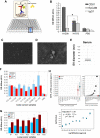
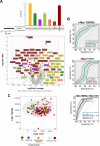
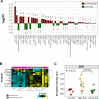
Extracellular vesicles (EVs) and their cargo represent an intriguing source of cancer biomarkers for developing robust and sensitive molecular tests by liquid biopsy. Prostate cancer (PCa) is still one of the most frequent and deadly tumor in men and analysis of EVs from biological fluids of PCa patients has proven the feasibility and the unprecedented potential of such an approach. Here, we exploited an antibody-based proteomic technology, i.e. the Reverse-Phase Protein microArrays (RPPA), to measure key antigens and activated signaling in EVs isolated from sera of PCa patients. Notably, we found tumor-specific protein profiles associated with clinical settings as well as candidate markers for EV-based tumor diagnosis. Among others, PD-L1, ERG, Integrin-β5, Survivin, TGF-β, phosphorylated-TSC2 as well as partners of the MAP-kinase and mTOR pathways emerged as differentially expressed endpoints in tumor-derived EVs. In addition, the retrospective analysis of EVs from a 15-year follow-up cohort generated a protein signature with prognostic significance. Our results confirm that serum-derived EV cargo may be exploited to improve the current diagnostic procedures while providing potential prognostic and predictive information. The approach proposed here has been already applied to tumor entities other than PCa, thus proving its value in translational medicine and paving the way to innovative, clinically meaningful tools.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
_targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer.Onco_target. 2017 Jan 17;8(3):4960-4976. doi: 10.18632/onco_target.13634. Onco_target. 2017. PMID: 27903962 Free PMC article.
-
Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer.Sci Rep. 2017 Feb 17;7:42961. doi: 10.1038/srep42961. Sci Rep. 2017. PMID: 28211531 Free PMC article.
-
Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis.Theranostics. 2020 Jan 16;10(5):2309-2326. doi: 10.7150/thno.39486. eCollection 2020. Theranostics. 2020. PMID: 32089744 Free PMC article. Review.
-
Proteomic characterization of circulating extracellular vesicles identifies novel serum myeloma associated markers.J Proteomics. 2016 Mar 16;136:89-98. doi: 10.1016/j.jprot.2015.12.016. Epub 2016 Jan 13. J Proteomics. 2016. PMID: 26775013 Free PMC article. Clinical Trial.
-
Extracellular vesicles for liquid biopsy in prostate cancer: where are we and where are we headed?Prostate Cancer Prostatic Dis. 2017 Sep;20(3):251-258. doi: 10.1038/pcan.2017.7. Epub 2017 Apr 4. Prostate Cancer Prostatic Dis. 2017. PMID: 28374743 Free PMC article. Review.
Cited by
-
Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology.Biomark Res. 2023 Nov 17;11(1):99. doi: 10.1186/s40364-023-00540-2. Biomark Res. 2023. PMID: 37978566 Free PMC article. Review.
-
On the Road to Accurate Protein Biomarkers in Prostate Cancer Diagnosis and Prognosis: Current Status and Future Advances.Int J Mol Sci. 2021 Dec 17;22(24):13537. doi: 10.3390/ijms222413537. Int J Mol Sci. 2021. PMID: 34948334 Free PMC article. Review.
-
Recent Advances in Blood-Based Liquid Biopsy Approaches in Prostate Cancer.Cancer J. 2023 Jul-Aug 01;29(4):220-225. doi: 10.1097/PPO.0000000000000672. Cancer J. 2023. PMID: 37471612 Free PMC article. Review.
-
Extracellular vesicles in neuroblastoma: role in progression, resistance to therapy and diagnostics.Front Immunol. 2024 Apr 9;15:1385875. doi: 10.3389/fimmu.2024.1385875. eCollection 2024. Front Immunol. 2024. PMID: 38660306 Free PMC article. Review.
-
The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery.Biomedicines. 2023 Dec 28;12(1):79. doi: 10.3390/biomedicines12010079. Biomedicines. 2023. PMID: 38255186 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Neppl-Huber C, Zappa M, Coebergh JW, Rapiti E, Rachtan J, Holleczek B, et al. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol. 2012;23:1325–34. doi: 10.1093/annonc/mdr414. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

