Effectiveness of consumer-grade contactless vital signs monitors: a systematic review and meta-analysis
- PMID: 34240262
- PMCID: PMC8266631
- DOI: 10.1007/s10877-021-00734-9
Effectiveness of consumer-grade contactless vital signs monitors: a systematic review and meta-analysis
Abstract
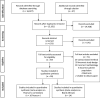
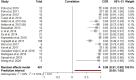
The objective of this systematic review and meta-analysis was to analyze the effectiveness of contactless vital sign monitors that utilize a consumer-friendly camera versus medical grade instruments. A multiple database search was conducted from inception to September 2020. Inclusion criteria were as follows: studies that used a consumer-grade camera (smartphone/webcam) to examine contactless vital signs in adults; evaluated the non-contact device against a reference medical device; and used the participants' face for measurement. Twenty-six studies were included in the review of which 16 were included in Pearson's correlation and 14 studies were included in the Bland-Altman meta-analysis. Twenty-two studies measured heart rate (HR) (92%), three measured blood pressure (BP) (12%), and respiratory rate (RR) (12%). No study examined blood oxygen saturation (SpO2). Most studies had a small sample size (≤ 30 participants) and were performed in a laboratory setting. Our meta-analysis found that consumer-grade contactless vital sign monitors were accurate in comparison to a medical device in measuring HR. Current contactless monitors have limitations such as motion, poor lighting, and lack of automatic face tracking. Currently available consumer-friendly contactless monitors measure HR accurately compared to standard medical devices. More studies are needed to assess the accuracy of contactless BP and RR monitors. Implementation of contactless vital sign monitors for clinical use will require validation in a larger population, in a clinical setting, and expanded to encompass other vital signs including BP, RR, and SpO2.
Keywords: Blood pressure; Camera; Contactless monitors; Heart rate; Photoplethysmography; Vital signs.
© 2021. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Frances Chung: Reports research support from University Health Network Foundation, Up-to-date royalties, consultant to Takeda Pharma, STOP-Bang proprietary to University Health Network. All other authors have no conflicts to disclose.
Figures

Similar articles
-
The Accuracy of Pulse Oxygen Saturation, Heart Rate, Blood Pressure, and Respiratory Rate Raised by a Contactless Telehealth Portal: Validation Study.JMIR Form Res. 2024 Jun 28;8:e55361. doi: 10.2196/55361. JMIR Form Res. 2024. PMID: 38598698 Free PMC article.
-
Continuous Monitoring of Vital Signs Using Cameras: A Systematic Review.Sensors (Basel). 2022 May 28;22(11):4097. doi: 10.3390/s22114097. Sensors (Basel). 2022. PMID: 35684717 Free PMC article. Review.
-
Contactless monitoring of respiratory rate (RR) and heart rate (HR) in non-acuity settings: a clinical validity study.BMJ Open. 2022 Dec 23;12(12):e065790. doi: 10.1136/bmjopen-2022-065790. BMJ Open. 2022. PMID: 36564107 Free PMC article.
-
Accuracy of Vital Signs Measurements by a Smartwatch and a Portable Health Device: Validation Study.JMIR Mhealth Uhealth. 2020 Feb 12;8(2):e16811. doi: 10.2196/16811. JMIR Mhealth Uhealth. 2020. PMID: 32049066 Free PMC article.
-
Novel wearable and contactless heart rate, respiratory rate, and oxygen saturation monitoring devices: a systematic review and meta-analysis.Anaesthesia. 2022 Nov;77(11):1268-1280. doi: 10.1111/anae.15834. Epub 2022 Aug 10. Anaesthesia. 2022. PMID: 35947876 Review.
Cited by
-
Can a specific biobehavioral-based therapeutic education program lead to changes in pain perception and brain plasticity biomarkers in chronic pain patients? A study protocol for a randomized clinical trial.PLoS One. 2024 Jan 19;19(1):e0289430. doi: 10.1371/journal.pone.0289430. eCollection 2024. PLoS One. 2024. PMID: 38241249 Free PMC article.
-
Accurate and Reliable Assessment of Heart Rate in Real-Life Clinical Settings Using an Imaging Photoplethysmography.J Clin Med. 2022 Oct 17;11(20):6101. doi: 10.3390/jcm11206101. J Clin Med. 2022. PMID: 36294422 Free PMC article.
-
Algoritmically improved microwave radar monitors breathing more acurrate than sensorized belt.Sci Rep. 2022 Aug 24;12(1):14412. doi: 10.1038/s41598-022-18808-2. Sci Rep. 2022. PMID: 36002632 Free PMC article.
-
Clinical applications of contactless photoplethysmography for monitoring in adults: A systematic review and meta-analysis.J Clin Transl Sci. 2023 May 15;7(1):e129. doi: 10.1017/cts.2023.547. eCollection 2023. J Clin Transl Sci. 2023. PMID: 37313385 Free PMC article. Review.
-
Performance of Contactless Respiratory Rate Monitoring by Albus HomeTM, an Automated System for Nocturnal Monitoring at Home: A Validation Study.Sensors (Basel). 2022 Sep 21;22(19):7142. doi: 10.3390/s22197142. Sensors (Basel). 2022. PMID: 36236241 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

