Interactions of cone cannabinoid CB1 and dopamine D4 receptors increase day/night difference in rod-cone gap junction coupling in goldfish retina
- PMID: 34252195
- PMCID: PMC8882046
- DOI: 10.1113/JP281308
Interactions of cone cannabinoid CB1 and dopamine D4 receptors increase day/night difference in rod-cone gap junction coupling in goldfish retina
Abstract
Key points: Although cone and rod photoreceptor cells in the retina have a type of cannabinoid receptor called a CB1 receptor, little is known about how cannabinoids, the active component in marijuana, affect retinal function. Studies have shown that a circadian (24-h) clock in the retina uses dopamine receptors, which are also on photoreceptors, to regulate gap junctions (a type of cell-to-cell communication) between rods and cones, so that they are functional (open) at night but closed in the day. We show that CB1 receptors have opposite effects on rod-cone gap junctions in day and night, decreasing communication in the day when dopamine receptors are active and increasing communication when dopamine receptors are inactive. CB1 and dopamine receptors thus work together to enhance the day/night difference in rod-cone gap junction communication. The increased rod-cone communication at night due to cannabinoid CB1 receptors may help improve night vision.
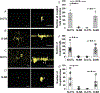
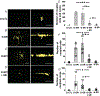
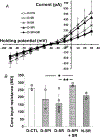
Abstract: Cannabinoid CB1 receptors and dopamine D4 receptors in the brain form receptor complexes that interact but the physiological function of these interactions in intact tissue remains unclear. In vertebrate retina, rods and cones, which are connected by gap junctions, express both CB1 and D4 receptors. Because the retinal circadian clock uses cone D4 receptors to decrease rod-cone gap junction coupling in the day and to increase it at night, we studied whether an interaction between cone CB1 and D4 receptors increases the day/night difference in rod-cone coupling compared to D4 receptors acting alone. Using electrical recording and injections of Neurobiotin tracer into individual cones in intact goldfish retinas, we found that SR141716A (a CB1 receptor antagonist) application alone in the day increased both the extent of rod-cone tracer coupling and rod input to cones, which reaches cones via open gap junctions. Conversely, SR141716A application alone at night or SR141716A application in the day following 30-min spiperone (a D4 receptor antagonist) application decreased both rod-cone tracer coupling and rod input to cones. These results show that endogenous activation of cone CB1 receptors decreases rod-cone coupling in the day when D4 receptors are activated but increases it at night when D4 receptors are not activated. Therefore, the D4 receptor-dependent day/night switch in the effects of CB1 receptor activation results in an enhancement of the day/night difference in rod-cone coupling. This synergistic interaction increases detection of very dim large objects at night and fine spatial details in the day.
Keywords: cannabinoid CB1 receptor; circadian rhythm; cone photoreceptor cell; dopamine D4 receptor; electrical synapse; gap junction; rod photoreceptor cell.
© 2021 The Authors. The Journal of Physiology © 2021 The Physiological Society.
Conflict of interest statement
Additional information
Conflict of interest statement
The authors declare no competing financial interests.
Figures
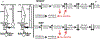



Similar articles
-
Circadian clock organization in the retina: From clock components to rod and cone pathways and visual function.Prog Retin Eye Res. 2023 May;94:101119. doi: 10.1016/j.preteyeres.2022.101119. Epub 2022 Dec 8. Prog Retin Eye Res. 2023. PMID: 36503722 Free PMC article. Review.
-
A Circadian Clock in the Retina Regulates Rod-Cone Gap Junction Coupling and Neuronal Light Responses via Activation of Adenosine A2A Receptors.Front Cell Neurosci. 2021 Jan 12;14:605067. doi: 10.3389/fncel.2020.605067. eCollection 2020. Front Cell Neurosci. 2021. PMID: 33510619 Free PMC article.
-
The circadian clock in the retina controls rod-cone coupling.Neuron. 2008 Sep 11;59(5):790-801. doi: 10.1016/j.neuron.2008.07.017. Neuron. 2008. PMID: 18786362 Free PMC article.
-
Analysis of rod/cone gap junctions from the reconstruction of mouse photoreceptor terminals.Elife. 2022 Apr 26;11:e73039. doi: 10.7554/eLife.73039. Elife. 2022. PMID: 35471186 Free PMC article.
-
When microscopy and electrophysiology meet connectomics-Steve Massey's contribution to unraveling the structure and function of the rod/cone gap junction.Front Ophthalmol (Lausanne). 2023 Nov 17;3:1305131. doi: 10.3389/fopht.2023.1305131. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983007 Free PMC article. Review.
Cited by
-
Increased H3K27 trimethylation contributes to cone survival in a mouse model of cone dystrophy.Cell Mol Life Sci. 2022 Jul 10;79(8):409. doi: 10.1007/s00018-022-04436-6. Cell Mol Life Sci. 2022. PMID: 35810394 Free PMC article.
-
Extrinsic and Intrinsic Factors Determine Expression Levels of Gap Junction-Forming Connexins in the Mammalian Retina.Biomolecules. 2023 Jul 13;13(7):1119. doi: 10.3390/biom13071119. Biomolecules. 2023. PMID: 37509155 Free PMC article. Review.
-
Circadian clock organization in the retina: From clock components to rod and cone pathways and visual function.Prog Retin Eye Res. 2023 May;94:101119. doi: 10.1016/j.preteyeres.2022.101119. Epub 2022 Dec 8. Prog Retin Eye Res. 2023. PMID: 36503722 Free PMC article. Review.
References
-
- Ariel M, Mangel SC & Dowling JE (1986). N-Methyl D-aspartate acts as an antagonist of the photoreceptor transmitter in the carp retina. Brain Res 372, 143–148. - PubMed
-
- Bagher AM, Laprairie RB, Kelly ME & Denovan-Wright EM (2016). Antagonism of dopamine receptor 2 long affects cannabinoid receptor 1 signaling in a cell culture model of striatal medium spiny projection neurons. Mol Pharmacol 89, 652–666. - PubMed
-
- Blazynski C (1990). Discrete distributions of adenosine receptors in mammalian retina. J Neurochem 54, 648–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

