Anoctamin1 Induces Hyperproliferation of HaCaT Keratinocytes and Triggers Imiquimod-Induced Psoriasis-Like Skin Injury in Mice
- PMID: 34281197
- PMCID: PMC8268182
- DOI: 10.3390/ijms22137145
Anoctamin1 Induces Hyperproliferation of HaCaT Keratinocytes and Triggers Imiquimod-Induced Psoriasis-Like Skin Injury in Mice
Abstract
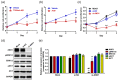
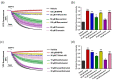
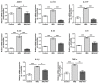
Psoriasis, a long-lasting and multifactorial skin disease, is related to comorbidities such as metabolic disease, depression, and psoriatic arthritis. Psoriasis occurs due to a variety of factors including keratinocyte hyperproliferation, inflammation, and abnormal differentiation. Proinflammatory cytokines upregulated by increased activation of keratinocytes and immune cells in the skin trigger progression of psoriasis. This study aimed to investigate the effects of anoctamin1 (ANO1) on psoriasis development in vitro and in vivo. We analyzed the proliferation of HaCaT keratinocytes and ANO1-related ERK and AKT signaling pathways after ANO1 inhibitor (T16Ainh-A01 and Ani9) treatment and knock-down of ANO1. Furthermore, after applying imiquimod (IMQ) cream or coapplying IMQ cream and T16Ainh-A01 on mouse ears, we not only observed psoriatic symptoms, including ear thickening, but also quantified the effects of treatment on ERK and AKT signaling-involved proteins and proinflammatory cytokines. Inhibition of ANO1 attenuated the proliferation of HaCaT cells and induced reduction of pERK1/2. Coapplication of IMQ and T16Ainh-A01 on ears of mice reduced not only symptoms of IMQ-induced psoriasis such as thickening and erythema, but also expression of ANO1 and pERK1/2 compared to that of application of IMQ alone. In addition, the expression levels of IL-17A, IL-17F, IL-22, IL-23, IL-6, IL-1β, and TNF-α increased after applying IMQ and were significantly reduced by coapplying IMQ and T16Ainh-A01. These results aid in understanding the underlying mechanisms of ANO1 in epidermal layer keratinocyte hyperproliferation and suggest the potential of ANO1 as a _target to treat psoriasis.
Keywords: ERK pathway; anoctamin 1; imiquimod; keratinocyte; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice.Biol Res. 2020 Oct 20;53(1):48. doi: 10.1186/s40659-020-00316-0. Biol Res. 2020. PMID: 33081840 Free PMC article.
-
The potential of Diosgenin in treating psoriasis: Studies from HaCaT keratinocytes and imiquimod-induced murine model.Life Sci. 2020 Jan 15;241:117115. doi: 10.1016/j.lfs.2019.117115. Epub 2019 Nov 29. Life Sci. 2020. PMID: 31790685
-
Gypenosides alleviates HaCaT keratinocyte hyperproliferation and ameliorates imiquimod-induced psoriasis in mice.Allergol Immunopathol (Madr). 2024 Nov 1;52(6):22-32. doi: 10.15586/aei.v52i6.1157. eCollection 2024. Allergol Immunopathol (Madr). 2024. PMID: 39515792
-
Cyclin-Dependent kinase 9 (CDK9) inhibitor Atuveciclib ameliorates Imiquimod-Induced Psoriasis-Like dermatitis in mice by inhibiting various inflammation factors via STAT3 signaling pathway.Int Immunopharmacol. 2024 Mar 10;129:111652. doi: 10.1016/j.intimp.2024.111652. Epub 2024 Feb 8. Int Immunopharmacol. 2024. PMID: 38335657 Review.
-
Flavonoid compounds and their synergistic effects: Promising approaches for the prevention and treatment of psoriasis with emphasis on keratinocytes - A systematic and mechanistic review.Int Immunopharmacol. 2024 Sep 10;138:112561. doi: 10.1016/j.intimp.2024.112561. Epub 2024 Jun 27. Int Immunopharmacol. 2024. PMID: 38941673 Review.
Cited by
-
Leucosceptoside A from Devil's Claw Modulates Psoriasis-like Inflammation via Suppression of the PI3K/AKT Signaling Pathway in Keratinocytes.Molecules. 2021 Nov 20;26(22):7014. doi: 10.3390/molecules26227014. Molecules. 2021. PMID: 34834106 Free PMC article.
-
Ca2+-Activated Chloride Channels and Phospholipid Scramblases.Int J Mol Sci. 2022 Feb 15;23(4):2158. doi: 10.3390/ijms23042158. Int J Mol Sci. 2022. PMID: 35216275 Free PMC article.
-
Pathophysiological Roles of Ion Channels in Epidermal Cells, Immune Cells, and Sensory Neurons in Psoriasis.Int J Mol Sci. 2024 Feb 27;25(5):2756. doi: 10.3390/ijms25052756. Int J Mol Sci. 2024. PMID: 38474002 Free PMC article. Review.
-
Metformin Inhibits HaCaT Cell Proliferation Under Hyperlipidemia Through Reducing Reactive Oxygen Species via FOXO3 Activation.Clin Cosmet Investig Dermatol. 2022 Jul 22;15:1403-1413. doi: 10.2147/CCID.S368845. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 35910506 Free PMC article.
-
ANO1-downregulation induced by schisandrathera D: a novel therapeutic _target for the treatment of prostate and oral cancers.Front Pharmacol. 2023 May 18;14:1163970. doi: 10.3389/fphar.2023.1163970. eCollection 2023. Front Pharmacol. 2023. PMID: 37274097 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

