Anemoside B4 inhibits enterovirus 71 propagation in mice through upregulating 14-3-3 expression and type I interferon responses
- PMID: 34321612
- PMCID: PMC8976028
- DOI: 10.1038/s41401-021-00733-1
Anemoside B4 inhibits enterovirus 71 propagation in mice through upregulating 14-3-3 expression and type I interferon responses
Abstract
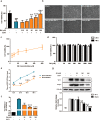
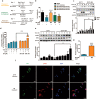
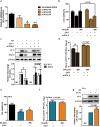
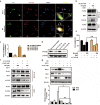
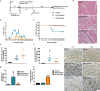
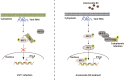
Enterovirus 71 (EV71) is the major pathogens of human hand, foot, and mouth disease (HFMD). EV71 efficiently escapes innate immunity responses of the host to cause infection. At present, no effective antiviral drugs for EV71 are available. Anemoside B4 (B4) is a natural saponin isolated from the roots of Pulsatilla chinensis (Bunge) Regel. P. chinensis extracts that shows a wide variety of biological activities. In this study, we investigated the antiviral activities of B4 against EV71 both in cell culture and in suckling mice. We showed that B4 (12.5-200 μM) dose dependently increased the viability of EV71-infected RD cells with an IC50 value of 24.95 ± 0.05 μM against EV71. The antiviral activity of B4 was associated with enhanced interferon (IFN)-β response, since knockdown of IFN-β abolished its antiviral activity. We also confirmed that the enhanced IFN response was mediated via activation of retinoic acid-inducible gene I (RIG-I) like receptors (RLRs) pathway, and it was executed by upregulation of 14-3-3 protein, which disrupted the interaction between yes-associated protein (YAP) and interferon regulatory factor 3 (IRF3). By using amino acids in cell culture (SILAC)-based proteomics profiling, we identified the Hippo pathway as the top-ranking functional cluster in B4-treated EV71-infected cells. In vivo experiments were conducted in suckling mice (2-day-old) infected with EV71 and subsequently B4 (200 mg · kg-1 · d-1, i.p.) was administered for 16 days. We showed that B4 administration effectively suppressed EV71 replication and improved muscle inflammation and limb activity. Meanwhile, B4 administration regulated the expressions of HFMD biomarkers IL-10 and IFN-γ, attenuating complications of EV71 infection. Collectively, our results suggest that B4 could enhance the antiviral effect of IFN-β by orchestrating Hippo and RLRs pathway, and B4 would be a potential lead compound for developing an anti-EV71 drug.
Keywords: 14-3-3 protein; Hippo pathway; anemoside B4; enterovirus 71; human hand, foot, and mouth disease; type I IFN.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Ginsenoside Rb1 is an immune-stimulatory agent with antiviral activity against enterovirus 71.J Ethnopharmacol. 2021 Feb 10;266:113401. doi: 10.1016/j.jep.2020.113401. Epub 2020 Sep 25. J Ethnopharmacol. 2021. PMID: 32980486
-
Downregulation of miR-155-5p facilitates enterovirus 71 replication through suppression of type I IFN response by _targeting FOXO3/IRF7 pathway.Cell Cycle. 2020 Jan;19(2):179-192. doi: 10.1080/15384101.2019.1704512. Epub 2019 Dec 19. Cell Cycle. 2020. PMID: 31856677 Free PMC article.
-
Toll-like receptor 9-mediated protection of enterovirus 71 infection in mice is due to the release of danger-associated molecular patterns.J Virol. 2014 Oct;88(20):11658-70. doi: 10.1128/JVI.00867-14. Epub 2014 Jul 30. J Virol. 2014. PMID: 25078697 Free PMC article.
-
Therapeutic potential of triterpenoid saponin anemoside B4 from Pulsatilla chinensis.Pharmacol Res. 2020 Oct;160:105079. doi: 10.1016/j.phrs.2020.105079. Epub 2020 Jul 15. Pharmacol Res. 2020. PMID: 32679180 Review.
-
Innate Immunity and Immune Evasion by Enterovirus 71.Viruses. 2015 Dec 14;7(12):6613-30. doi: 10.3390/v7122961. Viruses. 2015. PMID: 26694447 Free PMC article. Review.
Cited by
-
Anemoside B4, a new pyruvate carboxylase inhibitor, alleviates colitis by reprogramming macrophage function.Inflamm Res. 2024 Mar;73(3):345-362. doi: 10.1007/s00011-023-01840-x. Epub 2023 Dec 29. Inflamm Res. 2024. PMID: 38157008
-
Anemoside B4 inhibits SARS-CoV-2 replication in vitro and in vivo.Chin Herb Med. 2023 Dec 13;16(1):106-112. doi: 10.1016/j.chmed.2023.09.005. eCollection 2024 Jan. Chin Herb Med. 2023. PMID: 38375049 Free PMC article.
-
Construction of recombinant fluorescent LSDV for high-throughput screening of antiviral drugs.Vet Res. 2024 Mar 16;55(1):33. doi: 10.1186/s13567-024-01281-2. Vet Res. 2024. PMID: 38493160 Free PMC article.
-
Umbilical Cord Mesenchymal-Stem-Cell-Derived Exosomes Exhibit Anti-Oxidant and Antiviral Effects as Cell-Free Therapies.Viruses. 2023 Oct 15;15(10):2094. doi: 10.3390/v15102094. Viruses. 2023. PMID: 37896871 Free PMC article.
-
TBK1 and IRF3 are potential therapeutic _targets in Enterovirus A71-associated diseases.PLoS Negl Trop Dis. 2023 Jan 10;17(1):e0011001. doi: 10.1371/journal.pntd.0011001. eCollection 2023 Jan. PLoS Negl Trop Dis. 2023. PMID: 36626364 Free PMC article.
References
-
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10:778–90. - PubMed
-
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91–107. - PubMed
-
- Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD) Expert Rev Vaccines. 2016;15:599–606. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

