Obinutuzumab Plus Chemotherapy Compared with Rituximab Plus Chemotherapy in Previously Untreated Italian Patients with Advanced Follicular Lymphoma at Intermediate-High Risk: A Cost-Effectiveness Analysis
- PMID: 34321898
- PMCID: PMC8313400
- DOI: 10.2147/CEOR.S317885
Obinutuzumab Plus Chemotherapy Compared with Rituximab Plus Chemotherapy in Previously Untreated Italian Patients with Advanced Follicular Lymphoma at Intermediate-High Risk: A Cost-Effectiveness Analysis
Abstract
Objective: To assess the cost-effectiveness of obinutuzumab (O-chemo) in comparison to rituximab (R-chemo) in patients with untreated advanced follicular lymphoma (FL) at intermediate or high risk from an Italian National Health Service (NHS) perspective.
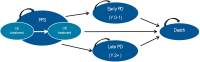
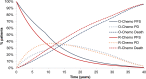
Methods: A previously developed four-state Markov model was adapted to estimate lifetime clinical outcomes and costs of Italian patients with advanced FL and an FL international predictive index score ≥2 in treatment with O-chemo and R-chemo. Life expectancy was derived from the GALLIUM and PRIMA clinical trials. Progression-free survival (PFS), early progressive disease (PD), and treatment duration were extrapolated by fitting parametric distributions to empirical data in GALLIUM and late PD to data in PRIMA. Expected survival was weighed by published utilities. Costs updated to 2020 Euros and health gains occurring after the first year were discounted at an annual 3% rate. Probabilistic sensitivity analysis (PSA) was carried out.
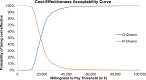
Results: O-chemo was associated with an incremental survival increase (0.97 life-years [LYs]), even when weighted for quality (0.88 quality-adjusted LYs [QALYs]), and incremental costs (around €15,000), driven by longer treatment during PFS state relative to R-chemo. The incremental cost-effectiveness ratio and incremental cost-utility ratio are both widely accepted by the Italian NHS (around €15,500/LY and €17,000/QALY gained, respectively). PSA simulations confirmed the robustness of results given sensible variations in assumptions.
Conclusion: O-chemo has superior clinical efficacy compared to rituximab, and should be considered a cost-effective option in first-line treatment of patients with advanced FL at intermediate or high risk in Italy. Incremental cost-effectiveness ratios are below the threshold considered affordable by developed countries.
Keywords: FLIPI score; ICER; PFS; QALY; economic evaluation; oncology.
© 2021 Bellone et al.
Conflict of interest statement
Dr Marco Bellone is an employee of AdRes, which has received project funding from Roche for the development of this research. Dr Marco Bellone reports grants from Roche during the study and grants from Roche, Sanofi, Janssen-Cilag Spa, Fresenius Kabi Deutschland, Novartis Farma, and Fresenius Kabi Italia outside the submitted work. Dr Lorenzo Pradelli is co-owner and an employee of AdRes, which has received project funding from Roche for the development of this research. Dr Lorenzo Pradelli report grants from Roche Spa during the study and grants from Janssen-Cilag, Sanofi, AstraZeneca, Biogen, and Medtronic outside the submitted work. Drs Stefano Molica and Adele Emanuela De Francesco are consultants for Roche. Drs Daniela Ghislieri, Emanuele Guardalben, and Antonietta Caputo are employees of Roche. The abstract of this paper was presented at the ISPOR Europe 2019 as a poster presentation, and can be viewed here: https://doi.org/10.1016/j.jval.2019.09.352
Figures

Similar articles
-
Cost-effectiveness of obinutuzumab versus rituximab biosimilars for previously untreated follicular lymphoma.J Manag Care Spec Pharm. 2021 May;27(5):615-624. doi: 10.18553/jmcp.2021.20424. Epub 2021 Feb 15. J Manag Care Spec Pharm. 2021. PMID: 33586513 Free PMC article.
-
Obinutuzumab in Combination with Chemotherapy for the First-Line Treatment of Patients with Advanced Follicular Lymphoma : An Evidence Review Group Evaluation of the NICE Single Technology Appraisal.Pharmacoeconomics. 2019 Aug;37(8):975-984. doi: 10.1007/s40273-018-0740-4. Pharmacoeconomics. 2019. PMID: 30547368 Free PMC article. Review.
-
Obinutuzumab plus chemotherapy followed by obinutuzumab monotherapy is cost-effective vs. rituximab plus chemotherapy followed by rituximab monotherapy for previously untreated follicular lymphoma patients in the United States.Leuk Lymphoma. 2019 Jul;60(7):1668-1676. doi: 10.1080/10428194.2018.1551532. Epub 2019 Jan 11. Leuk Lymphoma. 2019. PMID: 30632837 Clinical Trial.
-
Cost-effectiveness analysis of treatment regimens with obinutuzumab plus chemotherapy in Japan for untreated follicular lymphoma patients.J Med Econ. 2020 Oct;23(10):1130-1141. doi: 10.1080/13696998.2020.1791890. Epub 2020 Jul 23. J Med Econ. 2020. PMID: 32620061
-
Rituximab for the first-line treatment of stage III-IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic review and economic evaluation.Health Technol Assess. 2012;16(37):1-253, iii-iv. doi: 10.3310/hta16370. Health Technol Assess. 2012. PMID: 23021127 Review.
References
-
- Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795. doi:10.1002/ajh.24086 - DOI - PubMed
-
- Smith A, Crouch S, Howell D, Burton C, Patmore R, Roman E. Impact of age and socioeconomic status on treatment and survival from aggressive lymphoma: a UK population-based study of diffuse large B-cell lymphoma. Cancer Epidemiol. 2015;39(6):1103–1112. doi:10.1016/j.canep.2015.08.015 - DOI - PMC - PubMed
-
- European Medicines Agency. European Medicines Agency: public Summary of Opinion on Orphan Designation: obinutuzumab for the treatment of follicular lymphoma 23 July 2015. Available from: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/15/1504-p.... Accessed January, 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

