Methodology for the development of international clinical data standards for common cardiovascular conditions: European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart)
- PMID: 34351420
- PMCID: PMC9972518
- DOI: 10.1093/ehjqcco/qcab052
Methodology for the development of international clinical data standards for common cardiovascular conditions: European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart)
Abstract
Aims: Data standards are consensual specifications for the representation of data arising from different sources. If provided with internationally harmonized variables, permissible values, and clinical definitions, they have the potential to enable reliable between- and within-country analysis of care and outcomes. The European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart) is a European Society of Cardiology project that allows participating countries to collect patient data to undertake quality improvement, observational studies, drug and device surveillance, and registry-based randomized controlled trials for cardiovascular conditions. This paper describes the methodology for development of harmonized data standards for EuroHeart.
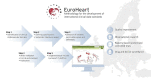
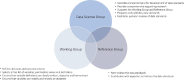
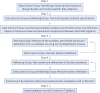
Methods and results: We adopted a five-step process for the development of harmonized data standards. The process includes (i) identification of clinical domains for data standard development by evaluating specific cardiovascular conditions with high prevalence and opportunities for quality improvement; (ii) construction of data standard specifications by systematic review of the literature; (iii) selection of variables by a domain-specific Working Group using a modified Delphi method; (iv) validation of data standards by a domain-specific Reference Group; and (v) implementation of the developed data standards into an IT platform.
Conclusion: This paper describes the approach adopted by EuroHeart for the development of clinical data standards for cardiovascular disease. The methodology has been developed and is used by EuroHeart to create a suite of international data standards for cardiovascular diseases. The EuroHeart data standards may be used to systematically capture individual patient data about clinical care and for research.
Keywords: Data definitions; Data standards; Data variables; EuroHeart; Methodology.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Similar articles
-
Data standards for heart failure: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart).Eur Heart J. 2022 Jun 14;43(23):2185-2195. doi: 10.1093/eurheartj/ehac151. Eur Heart J. 2022. PMID: 35443059 Free PMC article.
-
Data standards for atrial fibrillation/flutter and catheter ablation: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart).Eur Heart J Qual Care Clin Outcomes. 2023 Sep 12;9(6):609-620. doi: 10.1093/ehjqcco/qcac068. Eur Heart J Qual Care Clin Outcomes. 2023. PMID: 36243903 Free PMC article.
-
Data standards for transcatheter aortic valve implantation: the European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart).Eur Heart J Qual Care Clin Outcomes. 2023 Aug 7;9(5):529-536. doi: 10.1093/ehjqcco/qcac063. Eur Heart J Qual Care Clin Outcomes. 2023. PMID: 36195332 Free PMC article.
-
Data standards for acute coronary syndrome and percutaneous coronary intervention: the European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart).Eur Heart J. 2022 Jun 21;43(24):2269-2285. doi: 10.1093/eurheartj/ehac133. Eur Heart J. 2022. PMID: 35380662 Free PMC article. Review.
-
[Investigation methods in clinical cardiology. VI. Evaluation of results (outcomes) and its clinical relevance in cardiology: with a special reference to the quality of life].Rev Esp Cardiol. 1997 Mar;50(3):192-200. doi: 10.1016/s0300-8932(97)73203-2. Rev Esp Cardiol. 1997. PMID: 9132880 Review. Spanish.
Cited by
-
Data standards for heart failure: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart).Eur Heart J. 2022 Jun 14;43(23):2185-2195. doi: 10.1093/eurheartj/ehac151. Eur Heart J. 2022. PMID: 35443059 Free PMC article.
-
Quality and Utility of European Cardiovascular and Orthopaedic Registries for the Regulatory Evaluation of Medical Device Safety and Performance Across the Implant Lifecycle: A Systematic Review.Int J Health Policy Manag. 2023;12:7648. doi: 10.34172/ijhpm.2023.7648. Epub 2023 Jul 18. Int J Health Policy Manag. 2023. PMID: 37579359 Free PMC article.
-
Do we need to reconsider how we design and conduct randomized controlled trials?Eur Heart J Qual Care Clin Outcomes. 2022 Jun 6;8(4):374-376. doi: 10.1093/ehjqcco/qcac010. Eur Heart J Qual Care Clin Outcomes. 2022. PMID: 35175349 Free PMC article. No abstract available.
-
Data standards for atrial fibrillation/flutter and catheter ablation: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart).Eur Heart J Qual Care Clin Outcomes. 2023 Sep 12;9(6):609-620. doi: 10.1093/ehjqcco/qcac068. Eur Heart J Qual Care Clin Outcomes. 2023. PMID: 36243903 Free PMC article.
-
European Society of Cardiology quality indicators for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death.Europace. 2023 Feb 8;25(1):199-210. doi: 10.1093/europace/euac114. Europace. 2023. PMID: 36753478 Free PMC article. Review.
References
-
- Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held Cet al. . Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J 2017;38:3056–3065. - PMC - PubMed
-
- Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held Cet al. . Relations between implementation of new treatments and improved outcomes in patients with non-ST-elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J 2018;39:3766–3776. - PubMed
-
- Bebb O, Hall M, Fox KAA, Dondo TB, Timmis A, Bueno Het al. . Performance of hospitals according to the ESC ACCA quality indicators and 30-day mortality for acute myocardial infarction: national cohort study using the United Kingdom Myocardial Ischaemia National Audit Project (MINAP) register. Eur Heart J 2017;38:974–982. - PMC - PubMed