Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer's Disease Model
- PMID: 34356628
- PMCID: PMC8301787
- DOI: 10.3390/biom11071004
Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer's Disease Model
Abstract
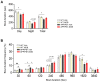
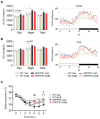
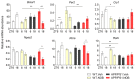
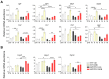
Alzheimer's disease (AD) is an age-related neurodegenerative disorder and the most common cause of dementia. Various pathogenic mechanisms have been proposed to contribute to disease progression, and recent research provided evidence linking dysregulated circadian rhythms/sleep and energy metabolism with AD. Previously, we found that the natural compound Nobiletin (NOB) can directly activate circadian cellular oscillators to promote metabolic health in disease models and healthy aging in naturally aged mice. In the current study, using the amyloid-β AD model APP/PS1, we investigated circadian, metabolic and amyloid characteristics of female mice and the effects of NOB. Female APP/PS1 mice showed reduced sleep bout duration, and NOB treatment exhibited a trend to improve it. While glucose tolerance was unchanged, female APP/PS1 mice displayed exaggerated oxygen consumption and CO2 production, which was mitigated by NOB. Likewise, cold tolerance in APP/PS1 was impaired relative to WT, and interestingly was markedly enhanced in NOB-treated APP/PS1 mice. Although circadian behavioral rhythms were largely unchanged, real-time qPCR analysis revealed altered expression of several core clock genes by NOB in the cerebral cortex, notably Bmal1, Npas2, and Rora. Moreover, NOB was also able to activate various clock-controlled metabolic genes involved in insulin signaling and mitochondrial function, including Igf1, Glut1, Insr, Irs1, Ucp2, and Ucp4. Finally, we observed that NOB attenuated the expression of several AD related genes including App, Bace1, and ApoE, reduced APP protein levels, and strongly ameliorated Aβ pathology in the cortex. Collectively, these results reveal novel genotype differences and importantly beneficial effects of a natural clock-enhancing compound in biological rhythms and related pathophysiology, suggesting the circadian clock as a modifiable _target for AD.
Keywords: Alzheimer’s disease; Nobiletin (NOB); amyloid beta (Aβ); circadian rhythms; energy metabolism; female APP/PS1 mice; mitochondria; sleep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The clock modulator Nobiletin mitigates astrogliosis-associated neuroinflammation and disease hallmarks in an Alzheimer's disease model.FASEB J. 2022 Mar;36(3):e22186. doi: 10.1096/fj.202101633R. FASEB J. 2022. PMID: 35120261 Free PMC article.
-
Chinese formula Guben-Jiannao Ye alleviates the dysfunction of circadian and sleep rhythms in APP/PS1 mice implicated in activation of the PI3K/AKT/mTOR signaling pathway.J Ethnopharmacol. 2024 Dec 5;335:118696. doi: 10.1016/j.jep.2024.118696. Epub 2024 Aug 14. J Ethnopharmacol. 2024. PMID: 39151711
-
Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APPxPS1 knock-in mice, a model for Alzheimer's disease.Exp Neurol. 2012 Aug;236(2):249-58. doi: 10.1016/j.expneurol.2012.05.011. Epub 2012 May 22. Exp Neurol. 2012. PMID: 22634208
-
Central and peripheral circadian clocks and their role in Alzheimer's disease.Dis Model Mech. 2017 Oct 1;10(10):1187-1199. doi: 10.1242/dmm.030627. Dis Model Mech. 2017. PMID: 28993311 Free PMC article. Review.
-
Mechanisms linking circadian clocks, sleep, and neurodegeneration.Science. 2016 Nov 25;354(6315):1004-1008. doi: 10.1126/science.aah4968. Science. 2016. PMID: 27885006 Free PMC article. Review.
Cited by
-
Circadian neurogenetics and its implications in neurophysiology, behavior, and chronomedicine.Neurosci Biobehav Rev. 2024 Feb;157:105523. doi: 10.1016/j.neubiorev.2023.105523. Epub 2023 Dec 22. Neurosci Biobehav Rev. 2024. PMID: 38142983 Review.
-
A Growing Link between Circadian Rhythms, Type 2 Diabetes Mellitus and Alzheimer's Disease.Int J Mol Sci. 2022 Jan 3;23(1):504. doi: 10.3390/ijms23010504. Int J Mol Sci. 2022. PMID: 35008933 Free PMC article. Review.
-
Natural Compounds for Preventing Age-Related Diseases and Cancers.Int J Mol Sci. 2024 Jul 9;25(14):7530. doi: 10.3390/ijms25147530. Int J Mol Sci. 2024. PMID: 39062777 Free PMC article. Review.
-
Modulation of the Circadian Rhythm and Oxidative Stress as Molecular _targets to Improve Vascular Dementia: A Pharmacological Perspective.Int J Mol Sci. 2024 Apr 16;25(8):4401. doi: 10.3390/ijms25084401. Int J Mol Sci. 2024. PMID: 38673986 Free PMC article. Review.
-
Involvement of Bile Acid Metabolism and Gut Microbiota in the Amelioration of Experimental Metabolism-Associated Fatty Liver Disease by Nobiletin.Molecules. 2024 Feb 23;29(5):976. doi: 10.3390/molecules29050976. Molecules. 2024. PMID: 38474489 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

