Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease
- PMID: 34408822
- PMCID: PMC8366131
- DOI: 10.1177/20406223211033119
Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease
Abstract
Background: The consistency in steatosis grading between magnetic resonance imaging-based proton density fat fraction (MRI-PDFF) and controlled attenuation parameter (CAP) before and after treatment remains unclear. This study aimed to compare the diagnostic accuracy of steatosis grading between MRI-PDFF and CAP using liver biopsy as standard and to evaluate the value of monitoring changes in steatosis grading with CAP during follow-up utilizing MRI-PDFF as a reference.
Methods: Consecutive patients from a biopsy cohort and a randomized controlled trial were included in this study and classified into 3 groups (the biopsy, orlistat treatment, and routine treatment subgroups). Hepatic steatosis was measured via MRI-PDFF and CAP at baseline and at the 6th month; the accuracy and cutoffs were assessed in the liver biopsy cohort at baseline.
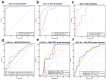
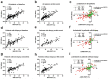
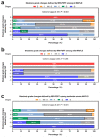
Results: A total of 209 consecutive patients were enrolled. MRI-PDFF and CAP showed comparable diagnostic accuracy for detecting pathological steatosis [⩾S1, area under the receiver operating characteristic curve (AUC) = 0.984 and 0.972, respectively]; in contrast, CAP presented significantly lower AUCs in grades S2-3 and S3 (0.820 and 0.815, respectively). The CAP values correlated well with the MRI-PDFF values at baseline and at the 6th month (r = 0.809 and 0.762, respectively, both p < 0.001), whereas a moderate correlation in their changes (r = 0.612 and 0.524 for moderate-severe and mild steatosis, respectively; both p < 0.001) was observed. The AUC of CAP change was obtained to predict MRI-PDFF changes of ⩾5% and ⩾10% (0.685 and 0.704, p < 0.001 and p = 0.001, respectively). The diagnostic agreement of steatosis grade changes between MRI-PDFF and CAP was weak (κ = 0.181, p = 0.001).
Conclusions: CAP has decreased value for the initial screening of moderate-severe steatosis and is limited in monitoring changes in steatosis during treatment. The confirmation of steatosis grading with MRI-PDFF remains necessary.
Keywords: controlled attenuation parameter; hepatic steatosis; liver biopsy; magnetic resonance imaging-based proton density fat fraction; metabolic associated fatty liver disease.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
Similar articles
-
MR Spectroscopy-derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic Steatosis.Radiology. 2018 Feb;286(2):547-556. doi: 10.1148/radiol.2017162931. Epub 2017 Sep 15. Radiology. 2018. PMID: 28915103
-
Prospective comparison of transient elastography, MRI and serum scores for grading steatosis and detecting non-alcoholic steatohepatitis in bariatric surgery candidates.JHEP Rep. 2021 Sep 30;3(6):100381. doi: 10.1016/j.jhepr.2021.100381. eCollection 2021 Dec. JHEP Rep. 2021. PMID: 34786549 Free PMC article.
-
Magnetic Resonance Imaging-Proton Density Fat Fraction vs. Transient Elastography-Controlled Attenuation Parameter in Diagnosing Non-alcoholic Fatty Liver Disease in Children and Adolescents: A Meta-Analysis of Diagnostic Accuracy.Front Pediatr. 2022 Jan 11;9:784221. doi: 10.3389/fped.2021.784221. eCollection 2021. Front Pediatr. 2022. PMID: 35087774 Free PMC article.
-
Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis.Eur Radiol. 2019 Jul;29(7):3564-3573. doi: 10.1007/s00330-019-06072-4. Epub 2019 Mar 21. Eur Radiol. 2019. PMID: 30899974 Review.
-
Diagnostic accuracy of ultrasound-guided attenuation parameter as a noninvasive test for steatosis in non-alcoholic fatty liver disease.J Med Ultrason (2001). 2021 Oct;48(4):471-480. doi: 10.1007/s10396-021-01123-0. Epub 2021 Aug 20. J Med Ultrason (2001). 2021. PMID: 34415481 Review.
Cited by
-
Machine learning prediction of hepatic steatosis using body composition parameters: A UK Biobank Study.NPJ Aging. 2024 Jan 9;10(1):4. doi: 10.1038/s41514-023-00127-z. NPJ Aging. 2024. PMID: 38195699 Free PMC article.
-
Non-Invasive Diagnostic of NAFLD in Type 2 Diabetes Mellitus and Risk Stratification: Strengths and Limitations.Life (Basel). 2023 Nov 27;13(12):2262. doi: 10.3390/life13122262. Life (Basel). 2023. PMID: 38137863 Free PMC article. Review.
-
First-in-human diagnostic study of hepatic steatosis with computed ultrasound tomography in echo mode.Commun Med (Lond). 2023 Dec 9;3(1):176. doi: 10.1038/s43856-023-00409-3. Commun Med (Lond). 2023. PMID: 38071269 Free PMC article.
-
Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal.Diagnostics (Basel). 2022 Sep 22;12(10):2287. doi: 10.3390/diagnostics12102287. Diagnostics (Basel). 2022. PMID: 36291976 Free PMC article. Review.
-
The Impact of an SGLT2 Inhibitor versus Ursodeoxycholic Acid on Liver Steatosis in Diabetic Patients.Pharmaceuticals (Basel). 2022 Dec 5;15(12):1516. doi: 10.3390/ph15121516. Pharmaceuticals (Basel). 2022. PMID: 36558967 Free PMC article.
References
-
- Younossi ZM, Koenig AB, Abdelatif D, et al.. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. - PubMed
-
- Bedossa P, Carrat F.Liver biopsy: the best, not the gold standard. J Hepatol 2009; 50: 1–3. - PubMed
-
- Raptis DA, Fischer MA, Graf R, et al.. MRI: the new reference standard in quantifying hepatic steatosis? Gut 2012; 61: 117–127. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

