Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial
- PMID: 34483029
- PMCID: PMC8384587
- DOI: 10.1016/j.jiac.2021.08.021
Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): A single-centre randomized, placebo-controlled trial
Abstract
Introduction: Ivermectin is an antiparasitic drug which has in-vitro efficacy in reducing severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) viral load. Hence, Ivermectin is under investigation as a repurposed agent for treating COVID-19.
Methods: In this pilot, double blind, randomized controlled trial, hospitalized patients with mild-to-moderate COVID-19 were assigned to a single oral administration of an elixir formulation of Ivermectin at either 24 mg or 12 mg dose, or placebo in a 1:1:1 ratio. The co-primary outcomes were conversion of RT-PCR to negative result and the decline of viral load at day 5 of enrolment. Safety outcomes included total and serious adverse events. The primary outcomes were assessed in patients who had positive RT-PCR at enrolment (modified intention-to-treat population). Safety outcomes were assessed in all patients who received the intervention (intention-to-treat population).
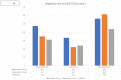
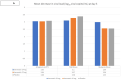
Results: Among the 157 patients randomized, 125 were included in modified intention-to-treat analysis. 40 patients each were assigned to Ivermectin 24 mg and 12 mg, and 45 patients to placebo. The RT-PCR negativity at day 5 was higher in the two Ivermectin arms but failed to attain statistical significance (Ivermectin 24 mg, 47.5%; 12 mg arm, 35.0%; and placebo arm, 31.1%; p-value = 0.30). The decline of viral load at day 5 was similar in each arm. No serious adverse events occurred.
Conclusions: In patients with mild and moderate COVID-19, a single oral administration of Ivermectin did not significantly increase either the negativity of RT-PCR or decline in viral load at day 5 of enrolment compared with placebo.
Keywords: COVID-19; Ivermectin; SARS-CoV-2; Viral load.
Copyright © 2021 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
None to declare.
Figures
Similar articles
-
Efficacy and safety of ivermectin in the treatment of mild to moderate COVID-19 infection: a randomized, double-blind, placebo-controlled trial.Trials. 2022 Aug 26;23(1):714. doi: 10.1186/s13063-022-06649-3. Trials. 2022. PMID: 36028897 Free PMC article. Clinical Trial.
-
High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial.Int J Antimicrob Agents. 2022 Feb;59(2):106516. doi: 10.1016/j.ijantimicag.2021.106516. Epub 2022 Jan 6. Int J Antimicrob Agents. 2022. PMID: 34999239 Free PMC article. Clinical Trial.
-
A randomized, double-blind study on the efficacy of oral domperidone versus placebo for reducing SARS-CoV-2 viral load in mild-to-moderate COVID-19 patients in primary health care.Ann Med. 2023;55(2):2268535. doi: 10.1080/07853890.2023.2268535. Epub 2023 Oct 17. Ann Med. 2023. PMID: 37847999 Free PMC article. Clinical Trial.
-
Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19.Am J Ther. 2021 Apr 22;28(3):e299-e318. doi: 10.1097/MJT.0000000000001377. Am J Ther. 2021. PMID: 34375047 Free PMC article. Review.
-
Meta-analysis of Randomized Trials of Ivermectin to Treat SARS-CoV-2 Infection.Open Forum Infect Dis. 2021 Jul 6;8(11):ofab358. doi: 10.1093/ofid/ofab358. eCollection 2021 Nov. Open Forum Infect Dis. 2021. Retraction in: Open Forum Infect Dis. 2022 Feb 05;9(3):ofac056. doi: 10.1093/ofid/ofac056 PMID: 34796244 Free PMC article. Retracted. Review.
Cited by
-
Ivermectin Should Not Be Recommended to Treat Severe Acute Respiratory Syndrome 2 Infection.Open Forum Infect Dis. 2021 Sep 2;8(12):ofab456. doi: 10.1093/ofid/ofab456. eCollection 2021 Dec. Open Forum Infect Dis. 2021. PMID: 34984211 Free PMC article. No abstract available.
-
Moxidectin and Ivermectin Inhibit SARS-CoV-2 Replication in Vero E6 Cells but Not in Human Primary Bronchial Epithelial Cells.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0154321. doi: 10.1128/AAC.01543-21. Epub 2021 Oct 11. Antimicrob Agents Chemother. 2022. PMID: 34633839 Free PMC article.
-
Clinical development of antivirals against SARS-CoV-2 and its variants.Curr Res Microb Sci. 2023 Dec 2;6:100208. doi: 10.1016/j.crmicr.2023.100208. eCollection 2024. Curr Res Microb Sci. 2023. PMID: 38149085 Free PMC article. Review.
-
Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants.Pharmaceuticals (Basel). 2022 Apr 2;15(4):445. doi: 10.3390/ph15040445. Pharmaceuticals (Basel). 2022. PMID: 35455442 Free PMC article.
-
Drug repositioning in the COVID-19 pandemic: fundamentals, synthetic routes, and overview of clinical studies.Eur J Clin Pharmacol. 2023 Jun;79(6):723-751. doi: 10.1007/s00228-023-03486-4. Epub 2023 Apr 20. Eur J Clin Pharmacol. 2023. PMID: 37081137 Free PMC article. Review.
References
-
- Omura S., Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014 Sep;30(9):445–455. - PubMed
-
- Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A., et al. The broad spectrum antiviral ivermectin _targets the host nuclear transport importin α/β1 heterodimer. Antivir Res. 2020;177:104760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous