Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields
- PMID: 34496717
- PMCID: PMC8437467
- DOI: 10.1080/21505594.2021.1960105
Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields
Abstract
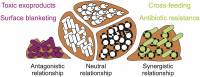
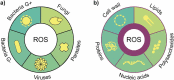
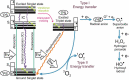
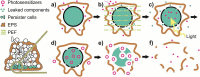
Currently, microbial biofilms have been the cause of a wide variety of infections in the human body, reaching 80% of all bacterial and fungal infections. The biofilms present specific properties that increase the resistance to antimicrobial treatments. Thus, the development of new approaches is urgent, and antimicrobial photodynamic therapy (aPDT) has been shown as a promising candidate. aPDT involves a synergic association of a photosensitizer (PS), molecular oxygen and visible light, producing highly reactive oxygen species (ROS) that cause the oxidation of several cellular components. This therapy attacks many components of the biofilm, including proteins, lipids, and nucleic acids present within the biofilm matrix; causing inhibition even in the cells that are inside the extracellular polymeric substance (EPS). Recent advances in designing new PSs to increase the production of ROS and the combination of aPDT with other therapies, especially pulsed electric fields (PEF), have contributed to enhanced biofilm inhibition. The PEF has proven to have antimicrobial effect once it is known that extensive chemical reactions occur when electric fields are applied. This type of treatment kills microorganisms not only due to membrane rupture but also due to the formation of reactive compounds including free oxygen, hydrogen, hydroxyl and hydroperoxyl radicals. So, this review aims to show the progress of aPDT and PEF against the biofilms, suggesting that the association of both methods can potentiate their effects and overcome biofilm infections.
Keywords: Antimicrobial resistance1; EPS3; PEF6; ROS4; aPDT5; biofilms2; photosensitizer7 and electroporation8.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Ultrasonic irradiation enhanced the efficacy of antimicrobial photodynamic therapy against methicillin-resistant Staphylococcus aureus biofilm.Ultrason Sonochem. 2023 Jul;97:106423. doi: 10.1016/j.ultsonch.2023.106423. Epub 2023 Apr 29. Ultrason Sonochem. 2023. PMID: 37235946 Free PMC article.
-
Theranostic nanoplatforms of emodin-chitosan with blue laser light on enhancing the anti-biofilm activity of photodynamic therapy against Streptococcus mutans biofilms on the enamel surface.BMC Microbiol. 2022 Mar 4;22(1):68. doi: 10.1186/s12866-022-02481-6. BMC Microbiol. 2022. PMID: 35246026 Free PMC article.
-
_targeted Antimicrobial Photodynamic Therapy of Biofilm-Embedded and Intracellular Staphylococci with a Phage Endolysin's Cell Binding Domain.Microbiol Spectr. 2022 Feb 23;10(1):e0146621. doi: 10.1128/spectrum.01466-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196798 Free PMC article.
-
Enhanced antimicrobial activity through the combination of antimicrobial photodynamic therapy and low-frequency ultrasonic irradiation.Adv Drug Deliv Rev. 2022 Apr;183:114168. doi: 10.1016/j.addr.2022.114168. Epub 2022 Feb 18. Adv Drug Deliv Rev. 2022. PMID: 35189265 Review.
-
Photodynamic therapy and combinatory treatments for the control of biofilm-associated infections.Lett Appl Microbiol. 2022 Sep;75(3):548-564. doi: 10.1111/lam.13762. Epub 2022 Jun 27. Lett Appl Microbiol. 2022. PMID: 35689422 Review.
Cited by
-
Postbiotic mediators derived from Lactobacillus species enhance riboflavin-mediated antimicrobial photodynamic therapy for eradication of Streptococcus mutans planktonic and biofilm growth.BMC Oral Health. 2024 Jul 24;24(1):836. doi: 10.1186/s12903-024-04620-z. BMC Oral Health. 2024. PMID: 39048998 Free PMC article.
-
Combating Drug-Resistant Bacteria Using Photothermally Active Nanomaterials: A Perspective Review.Front Microbiol. 2021 Nov 12;12:747019. doi: 10.3389/fmicb.2021.747019. eCollection 2021. Front Microbiol. 2021. PMID: 34867863 Free PMC article. Review.
-
Susceptibility of Dental Caries Microcosm Biofilms to Photodynamic Therapy Mediated by Fotoenticine.Pharmaceutics. 2021 Nov 10;13(11):1907. doi: 10.3390/pharmaceutics13111907. Pharmaceutics. 2021. PMID: 34834321 Free PMC article.
-
In Vitro Antimicrobial Photodynamic Therapy for Pseudomonas aeruginosa (P. aeruginosa) and methicillin-resistant Staphylococcus aureus (MRSA) Inhibition Using a Green Light Source.Pharmaceutics. 2024 Apr 9;16(4):518. doi: 10.3390/pharmaceutics16040518. Pharmaceutics. 2024. PMID: 38675180 Free PMC article.
-
Antimicrobial Photodynamic Therapy: Self-Disinfecting Surfaces for Controlling Microbial Infections.Microorganisms. 2024 Aug 1;12(8):1573. doi: 10.3390/microorganisms12081573. Microorganisms. 2024. PMID: 39203415 Free PMC article. Review.
References
-
- World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization. 2014. https://apps.who.int/iris/handle/10665/112642
-
- Smith R, Coast J.. The true cost of antimicrobial resistance. Bmj. 2013;346:f1493. - PubMed
-
- England PH.Keep antibiotics working [Online]. British society for antimicrobial therapy;2020. [cited 2020 May 14].
-
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2):114–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
