The reversed intra- and extracellular pH in tumors as a unified strategy to chemotherapeutic delivery using _targeted nanocarriers
- PMID: 34522586
- PMCID: PMC8424227
- DOI: 10.1016/j.apsb.2021.01.012
The reversed intra- and extracellular pH in tumors as a unified strategy to chemotherapeutic delivery using _targeted nanocarriers
Abstract
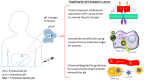
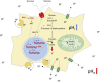
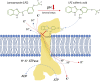
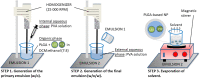
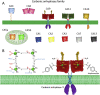
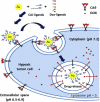
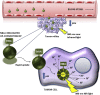
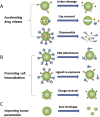
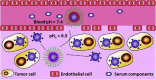
Solid tumors are complex entities, comprising a wide variety of malignancies with very different molecular alterations. Despite this, they share a set of characteristics known as "hallmarks of cancer" that can be used as common therapeutic _targets. Thus, every tumor needs to change its metabolism in order to obtain the energy levels required for its high proliferative rates, and these adaptations lead to alterations in extra- and intracellular pH. These changes in pH are common to all solid tumors, and can be used either as therapeutic _targets, blocking the cell proton transporters and reversing the pH changes, or as means to specifically deliver anticancer drugs. In this review we will describe how proton transport inhibitors in association with nanocarriers have been designed to block the pH changes that are needed for cancer cells to survive after their metabolic adaptations. We will also describe studies aiming to decrease intracellular pH in cancer using nanoparticles as molecular cages for protons which will be released upon UV or IR light exposure. Finally, we will comment on several studies that have used the extracellular pH in cancer for an enhanced cell internalization and tumor penetration of nanocarriers and a controlled drug delivery, describing how nanocarriers are being used to increase drug stability and specificity.
Keywords: Cancer; Chemotherapy; Metabolism of glucose; Proton transport inhibitors; Proton-caged carriers; _targeted drug delivery; Warburg effect; pH-Gradient inversion; pH-Sensitive nanocarriers.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
These authors have no conflicts of interest to declare in this work.
Figures
Similar articles
-
Advanced _targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance.Chem Soc Rev. 2013 Sep 7;42(17):7289-325. doi: 10.1039/c3cs60048c. Epub 2013 Apr 3. Chem Soc Rev. 2013. PMID: 23549663 Review.
-
Advancements in pH-responsive nanocarriers: enhancing drug delivery for tumor therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(11):1623-1642. doi: 10.1080/17425247.2023.2292678. Epub 2023 Dec 20. Expert Opin Drug Deliv. 2023. PMID: 38059646 Review.
-
Molecular machineries of pH dysregulation in tumor microenvironment: potential _targets for cancer therapy.Bioimpacts. 2017;7(2):115-133. doi: 10.15171/bi.2017.15. Epub 2017 Jun 7. Bioimpacts. 2017. PMID: 28752076 Free PMC article. Review.
-
Design of doxorubicin encapsulated pH-/thermo-responsive and cationic shell-crosslinked magnetic drug delivery system.Colloids Surf B Biointerfaces. 2022 Jan;209(Pt 2):112168. doi: 10.1016/j.colsurfb.2021.112168. Epub 2021 Oct 21. Colloids Surf B Biointerfaces. 2022. PMID: 34715504
Cited by
-
Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host-Guest Complexes in a Comparative Evaluation.Pharmaceutics. 2022 May 15;14(5):1059. doi: 10.3390/pharmaceutics14051059. Pharmaceutics. 2022. PMID: 35631645 Free PMC article.
-
Delivery of Chemotherapy Agents and Nucleic Acids with pH-Dependent Nanoparticles.Pharmaceutics. 2023 May 12;15(5):1482. doi: 10.3390/pharmaceutics15051482. Pharmaceutics. 2023. PMID: 37242725 Free PMC article. Review.
-
Cytotoxicity Enhancement in MCF-7 Breast Cancer Cells with Depolymerized Chitosan Delivery of α-Mangostin.Polymers (Basel). 2022 Aug 1;14(15):3139. doi: 10.3390/polym14153139. Polymers (Basel). 2022. PMID: 35956654 Free PMC article.
-
Peptide-Drug Conjugates: Design, Chemistry, and Drug Delivery System as a Novel Cancer Theranostic.ACS Pharmacol Transl Sci. 2024 Jan 24;7(2):309-334. doi: 10.1021/acsptsci.3c00269. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357281 Review.
-
Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis.Int J Mol Sci. 2023 Aug 10;24(16):12637. doi: 10.3390/ijms241612637. Int J Mol Sci. 2023. PMID: 37628818 Free PMC article.
References
-
- Harguindey S., Reshkin S.J. “The new pH-centric anticancer paradigm in Oncology and Medicine”; SCB, 2017. Semin Cancer Biol. 2017;43:1–4. - PubMed
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Bellou S., Pentheroudakis G., Murphy C., Fotsis T. Anti-angiogenesis in cancer therapy: hercules and hydra. Cancer Lett. 2013;338:219–228. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

