Cannabinoid receptor type 2 is upregulated in synovium following joint injury and mediates anti-inflammatory effects in synovial fibroblasts and macrophages
- PMID: 34537380
- PMCID: PMC8883578
- DOI: 10.1016/j.joca.2021.09.003
Cannabinoid receptor type 2 is upregulated in synovium following joint injury and mediates anti-inflammatory effects in synovial fibroblasts and macrophages
Abstract
Objective: Joint injury-induced perturbations to the endocannabinoid system (ECS), a regulator of both inflammation and nociception, remain largely uncharacterized. We employed a mouse model of ACL rupture to assess alterations to nociception, inflammation, and the ECS while using in vitro models to determine whether CB2 agonism can mitigate inflammatory signaling in macrophages and fibroblast-like synoviocytes (FLS).
Design: Mice underwent noninvasive ACL rupture (ACLR) via tibial compression-based loading. Nociception was measured longitudinally using mechanical allodynia and knee hyperalgesia testing. Synovitis was assessed using histological scoring and histomorphometry. Gene and protein markers of inflammation were characterized in whole joints and synovium. Immunohistochemistry assessed injury-induced alterations to CB1+, CB2+, and F4/80+ cells in synovium. To assess whether CB2 agonism can inhibit pro-inflammatory macrophage polarization, murine bone marrow-derived macrophages (mBMDM) were stimulated with IL-1β or conditioned medium from IL-1β-treated FLS and treated with vehicle (DMSO), the CB2 agonist HU308, or cannabidiol (CBD). Macrophage polarization was assessed as the ratio of M1-associated (IL1b, MMP1b, and IL6) to M2-associated (IL10, IL4, and CD206) gene expression. Human FLS (hFLS) isolated from synovial tissue of OA patients were treated with vehicle (DMSO) or HU308 following TNF-α or IL-1β stimulation to assess inhibition of catabolic/inflammatory gene expression.
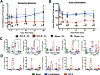
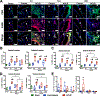
Results: ACLR induces synovitis, progressively-worsening PTOA severity, and an immediate and sustained increase in both mechanical allodynia and knee hyperalgesia, which persist beyond the resolution of molecular inflammation. Enrichment of CB2, but not CB1, was observed in ACLR synovium at 3d, 14d, and 28d, and CB2 was found to be associated with F4/80 (+) cells, which are increased in number in ACLR synovium at all time points. The CB2 agonist HU308 strongly inhibited mBMDM M1-type polarization following stimulation with either IL-1β or conditioned medium from IL-1β-treated mFLS, which was characterized by reductions in Il1b, Mmp1b, and Il6 and increases in Cd206 gene expression. Cannabidiol similarly inhibited IL-1β-induced mBMDM M1 polarization via a reduction in Il1b and an increase in Cd206 and Il4 gene expression. Lastly, in OA hFLS, HU308 treatment inhibited IL-1β-induced CCL2, MMP1, MMP3, and IL6 expression and further inhibited TNF-α-induced CCL2, MMP1, and GMCSF expression, demonstrating human OA-relevant anti-inflammatory effects by _targeting CB2.
Conclusions: Joint injury perturbs the intra-articular ECS, characterized by an increase in synovial F4/80(+) cells, which express CB2, but not CB1. _targeting CB2 in murine macrophages and human FLS induced potent anti-inflammatory and anti-catabolic effects, which indicates that the CB2 receptor plays a key role in regulating inflammatory signaling in the two primary effector cells in the synovium. The intraarticular ECS is therefore a potential therapeutic _target for blocking pathological inflammation in future disease-modifying PTOA treatments.
Keywords: Endocannabinoid system; Macrophage; Nociception; Post-traumatic osteoarthritis; Synovitis.
Copyright © 2021 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Figures

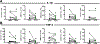
Similar articles
-
The Impact of the CB2 Cannabinoid Receptor in Inflammatory Diseases: An Update.Molecules. 2024 Jul 18;29(14):3381. doi: 10.3390/molecules29143381. Molecules. 2024. PMID: 39064959 Free PMC article. Review.
-
Sexual dimorphism of the synovial transcriptome underpins greater PTOA disease severity in male mice following joint injury.Osteoarthritis Cartilage. 2024 Sep;32(9):1060-1073. doi: 10.1016/j.joca.2023.07.012. Epub 2023 Sep 15. Osteoarthritis Cartilage. 2024. PMID: 37716404
-
Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis.Rheumatology (Oxford). 2014 May;53(5):802-9. doi: 10.1093/rheumatology/ket447. Epub 2014 Jan 17. Rheumatology (Oxford). 2014. PMID: 24440992
-
Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis.Arthritis Res Ther. 2008;10(2):R43. doi: 10.1186/ar2401. Epub 2008 Apr 16. Arthritis Res Ther. 2008. PMID: 18416822 Free PMC article.
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
Cited by
-
Exploring the versatile roles of the endocannabinoid system and phytocannabinoids in modulating bacterial infections.Infect Immun. 2024 Jun 11;92(6):e0002024. doi: 10.1128/iai.00020-24. Epub 2024 May 22. Infect Immun. 2024. PMID: 38775488 Free PMC article. Review.
-
Nanomaterials modulate tumor-associated macrophages for the treatment of digestive system tumors.Bioact Mater. 2024 Mar 20;36:376-412. doi: 10.1016/j.bioactmat.2024.03.003. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38544737 Free PMC article. Review.
-
The Impact of the CB2 Cannabinoid Receptor in Inflammatory Diseases: An Update.Molecules. 2024 Jul 18;29(14):3381. doi: 10.3390/molecules29143381. Molecules. 2024. PMID: 39064959 Free PMC article. Review.
-
Intra-articular sprouting of nociceptors accompanies progressive osteoarthritis: comparative evidence in four murine models.Front Neuroanat. 2024 Jul 15;18:1429124. doi: 10.3389/fnana.2024.1429124. eCollection 2024. Front Neuroanat. 2024. PMID: 39076825 Free PMC article.
-
Inflammation in osteoarthritis: the latest progress and ongoing challenges.Curr Opin Rheumatol. 2023 Mar 1;35(2):128-134. doi: 10.1097/BOR.0000000000000923. Epub 2022 Dec 22. Curr Opin Rheumatol. 2023. PMID: 36695054 Free PMC article. Review.
References
-
- Buckwalter JA, Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 28 (4), 1998, 192–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

