Methionine restriction exposes a _targetable redox vulnerability of triple-negative breast cancer cells by inducing thioredoxin reductase
- PMID: 34553295
- PMCID: PMC8793942
- DOI: 10.1007/s10549-021-06398-y
Methionine restriction exposes a _targetable redox vulnerability of triple-negative breast cancer cells by inducing thioredoxin reductase
Abstract
Purpose: Tumor cells are dependent on the glutathione and thioredoxin antioxidant pathways to survive oxidative stress. Since the essential amino acid methionine is converted to glutathione, we hypothesized that methionine restriction (MR) would deplete glutathione and render tumors dependent on the thioredoxin pathway and its rate-limiting enzyme thioredoxin reductase (TXNRD).
Methods: Triple (ER/PR/HER2)-negative breast cancer (TNBC) cells were treated with control or MR media and the effects on reactive oxygen species (ROS) and antioxidant signaling were examined. To determine the role of TXNRD in MR-induced cell death, TXNRD1 was inhibited by RNAi or the pan-TXNRD inhibitor auranofin, an antirheumatic agent. Metastatic and PDX TNBC mouse models were utilized to evaluate in vivo antitumor activity.
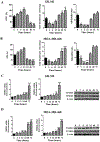
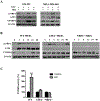
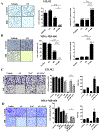
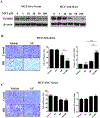
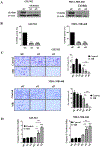
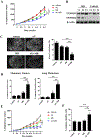
Results: MR rapidly and transiently increased ROS, depleted glutathione, and decreased the ratio of reduced glutathione/oxidized glutathione in TNBC cells. TXNRD1 mRNA and protein levels were induced by MR via a ROS-dependent mechanism mediated by the transcriptional regulators NRF2 and ATF4. MR dramatically sensitized TNBC cells to TXNRD1 silencing and the TXNRD inhibitor auranofin, as determined by crystal violet staining and caspase activity; these effects were suppressed by the antioxidant N-acetylcysteine. H-Ras-transformed MCF-10A cells, but not untransformed MCF-10A cells, were highly sensitive to the combination of auranofin and MR. Furthermore, dietary MR induced TXNRD1 expression in mammary tumors and enhanced the antitumor effects of auranofin in metastatic and PDX TNBC murine models.
Conclusion: MR exposes a vulnerability of TNBC cells to the TXNRD inhibitor auranofin by increasing expression of its molecular _target and creating a dependency on the thioredoxin pathway.
Keywords: Auranofin; Cancer; Glutathione; Methionine; Nutrition; Oxidative stress; Thioredoxin.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Lysine oxidase exposes a dependency on the thioredoxin antioxidant pathway in triple-negative breast cancer cells.Breast Cancer Res Treat. 2020 Oct;183(3):549-564. doi: 10.1007/s10549-020-05801-4. Epub 2020 Jul 21. Breast Cancer Res Treat. 2020. PMID: 32696316 Free PMC article.
-
Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti-PD-L1 antibody for treatment of triple-negative breast cancer.Int J Cancer. 2020 Jan 1;146(1):123-136. doi: 10.1002/ijc.32410. Epub 2019 May 31. Int J Cancer. 2020. PMID: 31090219
-
Inhibition of Thioredoxin/Thioredoxin Reductase Induces Synthetic Lethality in Lung Cancers with Compromised Glutathione Homeostasis.Cancer Res. 2019 Jan 1;79(1):125-132. doi: 10.1158/0008-5472.CAN-18-1938. Epub 2018 Nov 6. Cancer Res. 2019. PMID: 30401714 Free PMC article.
-
The thioredoxin antioxidant system.Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review.
-
Potential Anticancer Activity of Auranofin.Chem Pharm Bull (Tokyo). 2019;67(3):186-191. doi: 10.1248/cpb.c18-00767. Chem Pharm Bull (Tokyo). 2019. PMID: 30827998 Review.
Cited by
-
Ribonucleic Acid-Mediated Control of Protein Translation Under Stress.Antioxid Redox Signal. 2023 Aug;39(4-6):374-389. doi: 10.1089/ars.2023.0233. Antioxid Redox Signal. 2023. PMID: 37470212 Free PMC article. Review.
-
Characterization of methionine dependence in melanoma cells.Mol Omics. 2024 Jan 15;20(1):37-47. doi: 10.1039/d3mo00087g. Mol Omics. 2024. PMID: 37782107
-
Dietary Manipulation of Amino Acids for Cancer Therapy.Nutrients. 2023 Jun 25;15(13):2879. doi: 10.3390/nu15132879. Nutrients. 2023. PMID: 37447206 Free PMC article. Review.
-
Increased Response to Immune Checkpoint Inhibitors with Dietary Methionine Restriction in a Colorectal Cancer Model.Cancers (Basel). 2023 Sep 7;15(18):4467. doi: 10.3390/cancers15184467. Cancers (Basel). 2023. PMID: 37760436 Free PMC article.
-
Increased response to immune checkpoint inhibitors with dietary methionine restriction.bioRxiv [Preprint]. 2023 Apr 6:2023.04.05.535695. doi: 10.1101/2023.04.05.535695. bioRxiv. 2023. Update in: Cancers (Basel). 2023 Sep 07;15(18):4467. doi: 10.3390/cancers15184467 PMID: 37066240 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

