Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants
- PMID: 34639074
- PMCID: PMC8509322
- DOI: 10.3390/ijms221910733
Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants
Abstract
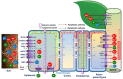
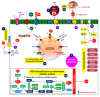
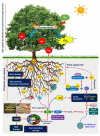
Soil salinization, which is aggravated by climate change and inappropriate anthropogenic activities, has emerged as a serious environmental problem, threatening sustainable agriculture and future food security. Although there has been considerable progress in developing crop varieties by introducing salt tolerance-associated traits, most crop cultivars grown in saline soils still exhibit a decline in yield, necessitating the search for alternatives. Halophytes, with their intrinsic salt tolerance characteristics, are known to have great potential in rehabilitating salt-contaminated soils to support plant growth in saline soils by employing various strategies, including phytoremediation. In addition, the recent identification and characterization of salt tolerance-related genes encoding signaling components from halophytes, which are naturally grown under high salinity, have paved the way for the development of transgenic crops with improved salt tolerance. In this review, we aim to provide a comprehensive update on salinity-induced negative effects on soils and plants, including alterations of physicochemical properties in soils, and changes in physiological and biochemical processes and ion disparities in plants. We also review the physiological and biochemical adaptation strategies that help halophytes grow and survive in salinity-affected areas. Furthermore, we illustrate the halophyte-mediated phytoremediation process in salinity-affected areas, as well as their potential impacts on soil properties. Importantly, based on the recent findings on salt tolerance mechanisms in halophytes, we also comprehensively discuss the potential of improving salt tolerance in crop plants by introducing candidate genes related to antiporters, ion transporters, antioxidants, and defense proteins from halophytes for conserving sustainable agriculture in salinity-prone areas.
Keywords: coastal areas; halophytes; phytoremediation; salt tolerance mechanisms; soil salinity; transgenic plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Potential use of halophytes to remediate saline soils.Biomed Res Int. 2014;2014:589341. doi: 10.1155/2014/589341. Epub 2014 Jul 6. Biomed Res Int. 2014. PMID: 25110683 Free PMC article. Review.
-
Plant salt tolerance: adaptations in halophytes.Ann Bot. 2015 Feb;115(3):327-31. doi: 10.1093/aob/mcu267. Ann Bot. 2015. PMID: 25844430 Free PMC article.
-
Understanding the salinity resilience and productivity of halophytes in saline environments.Plant Sci. 2024 Sep;346:112171. doi: 10.1016/j.plantsci.2024.112171. Epub 2024 Jul 3. Plant Sci. 2024. PMID: 38969140 Review.
-
Adaptation Mechanism of Salt Excluders under Saline Conditions and Its Applications.Int J Mol Sci. 2018 Nov 20;19(11):3668. doi: 10.3390/ijms19113668. Int J Mol Sci. 2018. PMID: 30463331 Free PMC article. Review.
-
Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model.Ann Bot. 2015 Feb;115(3):541-53. doi: 10.1093/aob/mcu194. Epub 2014 Oct 6. Ann Bot. 2015. PMID: 25288631 Free PMC article.
Cited by
-
Full-Length Transcriptome Analysis of the Halophyte Nitraria sibirica Pall.Genes (Basel). 2022 Apr 8;13(4):661. doi: 10.3390/genes13040661. Genes (Basel). 2022. PMID: 35456467 Free PMC article.
-
Chasing the mechanisms of ecologically adaptive salinity tolerance.Plant Commun. 2023 Nov 13;4(6):100571. doi: 10.1016/j.xplc.2023.100571. Epub 2023 Mar 7. Plant Commun. 2023. PMID: 36883005 Free PMC article. Review.
-
Genotypic-specific hormonal reprogramming and crosstalk are crucial for root growth and salt tolerance in bermudagrass (Cynodon dactylon).Front Plant Sci. 2022 Aug 4;13:956410. doi: 10.3389/fpls.2022.956410. eCollection 2022. Front Plant Sci. 2022. PMID: 35991415 Free PMC article.
-
Copper Oxide Nanoparticles Induced Growth and Physio-Biochemical Changes in Maize (Zea mays L.) in Saline Soil.Plants (Basel). 2024 Apr 11;13(8):1080. doi: 10.3390/plants13081080. Plants (Basel). 2024. PMID: 38674489 Free PMC article.
-
Detergent-Resistant Membranes in Chloroplasts and Mitochondria of the Halophyte Salicornia perennans under Salt Stress.Plants (Basel). 2023 Mar 10;12(6):1265. doi: 10.3390/plants12061265. Plants (Basel). 2023. PMID: 36986953 Free PMC article.
References
-
- Qadir M., Quillérou E., Nangia V., Murtaza G., Singh M., Thomas R.J., Drechsel P., Noble A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014;38:282–295. doi: 10.1111/1477-8947.12054. - DOI
-
- Minhas P., Qadir M., Yadav R. Groundwater irrigation induced soil sodification and response options. Agric. Water Manag. 2019;215:74–85. doi: 10.1016/j.agwat.2018.12.030. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

