Efficacy and safety of two artificial saliva-based polymers containing 0.1% pilocarpine for treatment of xerostomia: A randomized clinical pilot trial
- PMID: 34667494
- PMCID: PMC8501859
- DOI: 10.4317/jced.58415
Efficacy and safety of two artificial saliva-based polymers containing 0.1% pilocarpine for treatment of xerostomia: A randomized clinical pilot trial
Abstract
Background: Topical agents are the mainstay in the treatment of xerostomia, a common complaint most frequently associated with salivary dysfunction. This study aimed to compared the efficacy and safety for xerostomia treatment of 2 artificial saliva preparations containing 0.1% pilocarpine, and, either sodium carboxymethylcellulose (SCMC), or, sodium polyacrylate (SPA).
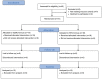
Material and methods: Thirty-one xerostomia patients were randomly allocated into either a SCMC-treated group (15 patients), or, a SPA-treated group (16 patients). The formulations were taken 0.5 ml, 4 times daily for 6 weeks and double-blinded assessed before and after treatments using Xerostomia Inventory (XI) and Clinical Oral Dryness Score (CODs). Unstimulated and stimulated whole salivary flow rates were measured.
Results: After treatment, the SCMC-treated group had significantly lower CODs and higher unstimulated and stimulated whole salivary flow rates (p<0.001, p=0.035, and p=0.013, respectively), while the SPA-treated group showed significantly lower CODs only (p=0.004). In contrast, SCMC-treated and SPA-treated groups at the 6th week after treatments showed non-significant differences in all assessments (p>0.05, all). Some adverse events (AEs) were reported, e.g., burning tongue, dizziness and watery eyes, but no severe AEs.
Conclusions: This randomized controlled pilot trial demonstrated superior efficacy of SCMC-formula over a SPA-formula after 6 weeks of xerostomia treatment. These formulations with topical pilocarpine proved safe in clinical use with minimal reported AE. Key words:Xerostomia, artificial saliva, sodium carboxymethylcellulose, sodium polyacrylate, pilocarpine.
Copyright: © 2021 Medicina Oral S.L.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Pilocarpine and artificial saliva for the treatment of xerostomia and xerophthalmia in Sjögren syndrome: a double-blind randomized controlled trial.Br J Dermatol. 2018 Nov;179(5):1056-1061. doi: 10.1111/bjd.16442. Epub 2018 May 29. Br J Dermatol. 2018. PMID: 29432648 Clinical Trial.
-
Effect of 0.1% pilocarpine mouthwash on xerostomia: double-blind, randomised controlled trial.J Oral Rehabil. 2014 Mar;41(3):226-35. doi: 10.1111/joor.12127. Epub 2013 Dec 30. J Oral Rehabil. 2014. PMID: 24527846 Clinical Trial.
-
Oral pilocarpine for radiation-induced xerostomia: integrated efficacy and safety results from two prospective randomized clinical trials.Int J Radiat Oncol Biol Phys. 1995 Feb 1;31(3):661-9. doi: 10.1016/0360-3016(94)00361-N. Int J Radiat Oncol Biol Phys. 1995. PMID: 7852133 Clinical Trial.
-
Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia.Drugs. 1995 Jan;49(1):143-55. doi: 10.2165/00003495-199549010-00010. Drugs. 1995. PMID: 7705213 Review.
-
Current therapies for xerostomia and salivary gland hypofunction associated with cancer therapies.Support Care Cancer. 2003 Apr;11(4):226-31. doi: 10.1007/s00520-002-0409-5. Epub 2002 Oct 22. Support Care Cancer. 2003. PMID: 12673460 Review.
Cited by
-
A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development.Pharmaceuticals (Basel). 2022 Jun 18;15(6):762. doi: 10.3390/ph15060762. Pharmaceuticals (Basel). 2022. PMID: 35745681 Free PMC article. Review.
-
Comparison of the 1 and 2% pilocarpine mouthwash in a xerostomic population: a randomized clinical trial.BMC Oral Health. 2022 Dec 1;22(1):548. doi: 10.1186/s12903-022-02576-6. BMC Oral Health. 2022. PMID: 36457091 Free PMC article. Clinical Trial.
-
Xerostomia: Advances and Challenges in Drug Development.Curr Drug _targets. 2024;25(5):301-305. doi: 10.2174/0113894501293941240228050343. Curr Drug _targets. 2024. PMID: 38424432 No abstract available.
References
-
- Saleh J, Figueiredo MAZ, Cherubini K, Salum FG. Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Arch Oral Biol. 2015;60:242–55. - PubMed
-
- Villa A, Wolff A, Aframian D, Vissink A, Ekström J, Proctor G. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction: prevalence, diagnosis, and treatment. Clin Oral Investig. 2015;19:1563–80. - PubMed
-
- Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55:238–44. - PubMed
-
- Millsop JW, Wang EA, Fazel N. Etiology, evaluation, and management of xerostomia. Clin Dermatol. 2017;35:468–76. - PubMed
LinkOut - more resources
Full Text Sources