Molecular Evolution of the Influenza A Virus Non-structural Protein 1 in Interspecies Transmission and Adaptation
- PMID: 34671321
- PMCID: PMC8521145
- DOI: 10.3389/fmicb.2021.693204
Molecular Evolution of the Influenza A Virus Non-structural Protein 1 in Interspecies Transmission and Adaptation
Abstract
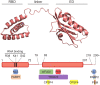
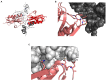
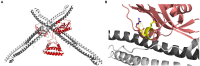
The non-structural protein 1 (NS1) of influenza A viruses plays important roles in viral fitness and in the process of interspecies adaptation. It is one of the most polymorphic and mutation-tolerant proteins of the influenza A genome, but its evolutionary patterns in different host species and the selective pressures that underlie them are hard to define. In this review, we highlight some of the species-specific molecular signatures apparent in different NS1 proteins and discuss two functions of NS1 in the process of viral adaptation to new host species. First, we consider the ability of NS1 proteins to broadly suppress host protein expression through interaction with CPSF4. This NS1 function can be spontaneously lost and regained through mutation and must be balanced against the need for host co-factors to aid efficient viral replication. Evidence suggests that this function of NS1 may be selectively lost in the initial stages of viral adaptation to some new host species. Second, we explore the ability of NS1 proteins to inhibit antiviral interferon signaling, an essential function for viral replication without which the virus is severely attenuated in any host. Innate immune suppression by NS1 not only enables viral replication in tissues, but also dampens the adaptive immune response and immunological memory. NS1 proteins suppress interferon signaling and effector functions through a variety of protein-protein interactions that may differ from host to host but must achieve similar goals. The multifunctional influenza A virus NS1 protein is highly plastic, highly versatile, and demonstrates a diversity of context-dependent solutions to the problem of interspecies adaptation.
Keywords: CPSF; RIG-I; adaptation; evolution; host; innate; interferon; species.
Copyright © 2021 Evseev and Magor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Avian Influenza NS1 Proteins Inhibit Human, but Not Duck, RIG-I Ubiquitination and Interferon Signaling.J Virol. 2022 Sep 28;96(18):e0077622. doi: 10.1128/jvi.00776-22. Epub 2022 Sep 7. J Virol. 2022. PMID: 36069546 Free PMC article.
-
Mammalian Adaptation of an Avian Influenza A Virus Involves Stepwise Changes in NS1.J Virol. 2018 Feb 12;92(5):e01875-17. doi: 10.1128/JVI.01875-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237841 Free PMC article.
-
The Nonstructural NS1 Protein of Influenza Viruses Modulates TP53 Splicing through Host Factor CPSF4.J Virol. 2019 Mar 21;93(7):e02168-18. doi: 10.1128/JVI.02168-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651364 Free PMC article.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
The multifunctional NS1 protein of influenza A viruses.J Gen Virol. 2008 Oct;89(Pt 10):2359-2376. doi: 10.1099/vir.0.2008/004606-0. J Gen Virol. 2008. PMID: 18796704 Review.
Cited by
-
Pro-Inflammatory Cytokines and Interferon-Stimulated Gene Responses Induced by Seasonal Influenza A Virus with Varying Growth Capabilities in Human Lung Epithelial Cell Lines.Vaccines (Basel). 2022 Sep 9;10(9):1507. doi: 10.3390/vaccines10091507. Vaccines (Basel). 2022. PMID: 36146585 Free PMC article.
-
Functional Analysis of GRSF1 in the Nuclear Export and Translation of Influenza A Virus mRNAs.Viruses. 2024 Jul 16;16(7):1136. doi: 10.3390/v16071136. Viruses. 2024. PMID: 39066299 Free PMC article.
-
Role of CIV NS1 Protein in Innate Immunity and Viral Replication.Int J Mol Sci. 2023 Jun 13;24(12):10056. doi: 10.3390/ijms241210056. Int J Mol Sci. 2023. PMID: 37373204 Free PMC article.
-
Antiviral responses versus virus-induced cellular shutoff: a game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1.Front Cell Infect Microbiol. 2024 Feb 5;14:1357866. doi: 10.3389/fcimb.2024.1357866. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38375361 Free PMC article. Review.
-
Energy landscape reshaped by strain-specific mutations underlies epistasis in NS1 evolution of influenza A virus.Nat Commun. 2022 Oct 1;13(1):5775. doi: 10.1038/s41467-022-33554-9. Nat Commun. 2022. PMID: 36182933 Free PMC article.
References
-
- Aragón T., de la Luna S., Novoa I., Carrasco L., Ortín J., Nieto A. (2000). Eukaryotic translation initiation factor 4GI is a cellular _target for NS1 protein, a translational activator of influenza virus. Mol. Cell. Biol. 20, 6259–6268. doi: 10.1128/MCB.20.17.6259-6268.2000, PMID: - DOI - PMC - PubMed
-
- Baskin C. R., Bielefeldt-Ohmann H., García-Sastre A., Tumpey T. M., Van Hoeven N., Carter V. S., et al. . (2007). Functional genomic and serological analysis of the protective immune response resulting from vaccination of macaques with an NS1-truncated influenza virus. J. Virol. 81, 11817–11827. doi: 10.1128/JVI.00590-07, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

