Synergism of a Novel 1,2,4-oxadiazole-containing Derivative with Oxacillin against Methicillin-Resistant Staphylococcus aureus
- PMID: 34680838
- PMCID: PMC8532612
- DOI: 10.3390/antibiotics10101258
Synergism of a Novel 1,2,4-oxadiazole-containing Derivative with Oxacillin against Methicillin-Resistant Staphylococcus aureus
Abstract
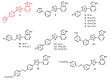
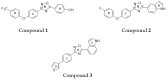
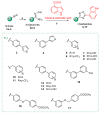
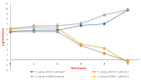
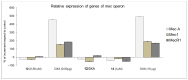
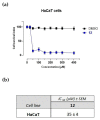
Staphylococcusaureus is an important opportunistic pathogen that causes many infections in humans and animals. The inappropriate use of antibiotics has favored the diffusion of methicillin-resistant S. aureus (MRSA), nullifying the efforts undertaken in the discovery of antimicrobial agents. Oxadiazole heterocycles represent privileged scaffolds for the development of new drugs because of their unique bioisosteric properties, easy synthesis, and therapeutic potential. A vast number of oxadiazole-containing derivatives have been discovered as potent antibacterial agents against multidrug-resistant MRSA strains. Here, we investigate the ability of a new library of oxadiazoles to contrast the growth of Gram-positive and Gram-negative strains. The strongest antimicrobial activity was obtained with compounds 3 (4 µM) and 12 (2 µM). Compound 12, selected for further evaluation, was found to be noncytotoxic on the HaCaT cell line up to 25 µM, bactericidal, and was able to improve the activity of oxacillin against the MRSA. The highest synergistic interaction was obtained with the combination values of 0.78 μM for compound 12, and 0.06 μg/mL for oxacillin. The FIC index value of 0.396 confirms the synergistic effect of compound 12 and oxacillin. MRSA treatment with compound 12 reduced the expression of genes included in the mec operon. In conclusion, 12 inhibited the growth of the MRSA and restored the activity of oxacillin, thus resulting in a promising compound in the treatment of MRSA infection.
Keywords: 1,2,4-oxadiazole; MRSA; Staphylococcus aureus; antimicrobial activity; drug discovery; structure–activity relationship; synergistic interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synergistic antibacterial effects of herbal extracts and antibiotics on methicillin-resistant Staphylococcus aureus: A computational and experimental study.Exp Biol Med (Maywood). 2017 Apr;242(7):731-743. doi: 10.1177/1535370216689828. Epub 2017 Jan 1. Exp Biol Med (Maywood). 2017. PMID: 28118725 Free PMC article.
-
New erythromycin derivatives enhance β-lactam antibiotics against methicillin-resistant Staphylococcus aureus.Lett Appl Microbiol. 2015 Apr;60(4):352-8. doi: 10.1111/lam.12378. Epub 2015 Jan 14. Lett Appl Microbiol. 2015. PMID: 25588530
-
Synergistic Effects Between Thioxanthones and Oxacillin Against Methicillin-Resistant Staphylococcus aureus.Microb Drug Resist. 2015 Aug;21(4):404-15. doi: 10.1089/mdr.2014.0162. Epub 2015 Mar 19. Microb Drug Resist. 2015. PMID: 25789724
-
A key review on oxadiazole analogs as potential methicillin-resistant Staphylococcus aureus (MRSA) activity: Structure-activity relationship studies.Eur J Med Chem. 2021 Jul 5;219:113442. doi: 10.1016/j.ejmech.2021.113442. Epub 2021 Apr 17. Eur J Med Chem. 2021. PMID: 33878562 Review.
-
1,2,4-Oxadiazole as a potential scaffold in agrochemistry: a review.Org Biomol Chem. 2023 Sep 27;21(37):7511-7524. doi: 10.1039/d3ob00934c. Org Biomol Chem. 2023. PMID: 37671568 Review.
Cited by
-
Unveiling the modulation of Pseudomonas aeruginosa virulence and biofilm formation by selective histone deacetylase 6 inhibitors.Front Microbiol. 2024 Feb 2;15:1340585. doi: 10.3389/fmicb.2024.1340585. eCollection 2024. Front Microbiol. 2024. PMID: 38371939 Free PMC article.
-
A Review on Five and Six-Membered Heterocyclic Compounds _targeting the Penicillin-Binding Protein 2 (PBP2A) of Methicillin-Resistant Staphylococcus aureus (MRSA).Molecules. 2023 Oct 10;28(20):7008. doi: 10.3390/molecules28207008. Molecules. 2023. PMID: 37894491 Free PMC article. Review.
References
-
- Laupland K.B., Lyytikainen O., Sogaard M., Kennedy K.J., Knudsen J.D., Ostergaard C., Galbraith J.C., Valiquette L., Jacobsson G., Collignon P., et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: A multinational population-based surveillance study. Clin. Microbiol. Infect. 2013;19:465–471. doi: 10.1111/j.1469-0691.2012.03903.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

