Atractylodin Suppresses TGF-β-Mediated Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells and Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice
- PMID: 34681813
- PMCID: PMC8570326
- DOI: 10.3390/ijms222011152
Atractylodin Suppresses TGF-β-Mediated Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells and Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice
Abstract
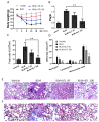
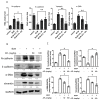
Idiopathic pulmonary fibrosis (IPF) is characterized by fibrotic change in alveolar epithelial cells and leads to the irreversible deterioration of pulmonary function. Transforming growth factor-beta 1 (TGF-β1)-induced epithelial-mesenchymal transition (EMT) in type 2 lung epithelial cells contributes to excessive collagen deposition and plays an important role in IPF. Atractylodin (ATL) is a kind of herbal medicine that has been proven to protect intestinal inflammation and attenuate acute lung injury. Our study aimed to determine whether EMT played a crucial role in the pathogenesis of pulmonary fibrosis and whether EMT can be utilized as a therapeutic _target by ATL treatment to mitigate IPF. To address this topic, we took two steps to investigate: 1. Utilization of anin vitro EMT model by treating alveolar epithelial cells (A549 cells) with TGF-β1 followed by ATL treatment for elucidating the underlying pathways, including Smad2/3 hyperphosphorylation, mitogen-activated protein kinase (MAPK) pathway overexpression, Snail and Slug upregulation, and loss of E-cadherin. Utilization of an in vivo lung injury model by treating bleomycin on mice followed by ATL treatment to demonstrate the therapeutic effectiveness, such as, less collagen deposition and lower E-cadherin expression. In conclusion, ATL attenuates TGF-β1-induced EMT in A549 cells and bleomycin-induced pulmonary fibrosis in mice.
Keywords: MAPK; Smad2/3; atractylodin; epithelial-mesenchymal transition; idiopathic pulmonary fibrosis; transforming growth factor-beta 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Osteopontin silencing attenuates bleomycin-induced murine pulmonary fibrosis by regulating epithelial-mesenchymal transition.Biomed Pharmacother. 2021 Jul;139:111633. doi: 10.1016/j.biopha.2021.111633. Epub 2021 May 8. Biomed Pharmacother. 2021. PMID: 34243624
-
Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway.Acta Pharmacol Sin. 2016 Jun;37(6):794-804. doi: 10.1038/aps.2016.36. Epub 2016 May 2. Acta Pharmacol Sin. 2016. PMID: 27133302 Free PMC article.
-
Tetraspanin 1 as a mediator of fibrosis inhibits EMT process and Smad2/3 and beta-catenin pathway in human pulmonary fibrosis.J Cell Mol Med. 2019 May;23(5):3583-3596. doi: 10.1111/jcmm.14258. Epub 2019 Mar 14. J Cell Mol Med. 2019. PMID: 30869194 Free PMC article.
-
Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway.Int J Mol Sci. 2021 Jun 5;22(11):6107. doi: 10.3390/ijms22116107. Int J Mol Sci. 2021. PMID: 34198949 Free PMC article. Review.
-
Cytokines as drivers: Unraveling the mechanisms of epithelial-mesenchymal transition in COVID-19 lung fibrosis.Biochem Biophys Res Commun. 2023 Dec 17;686:149118. doi: 10.1016/j.bbrc.2023.10.050. Epub 2023 Oct 14. Biochem Biophys Res Commun. 2023. PMID: 37931361 Review.
Cited by
-
Authenticating the geographic origins of Atractylodes lancea rhizome chemotypes in China through metabolite marker identification.Front Plant Sci. 2023 Sep 28;14:1237800. doi: 10.3389/fpls.2023.1237800. eCollection 2023. Front Plant Sci. 2023. PMID: 37841605 Free PMC article.
-
Arbutin alleviates Mycoplasma gallinarum-induced damage caused by pulmonary fibrosis via the JAK2/STAT3 pathway.Poult Sci. 2024 Dec;103(12):104434. doi: 10.1016/j.psj.2024.104434. Epub 2024 Oct 19. Poult Sci. 2024. PMID: 39467406 Free PMC article.
-
Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis.Adv Respir Med. 2023 Jan 31;91(1):26-48. doi: 10.3390/arm91010005. Adv Respir Med. 2023. PMID: 36825939 Free PMC article. Review.
-
A systematic review of the research progress of traditional Chinese medicine against pulmonary fibrosis: from a pharmacological perspective.Chin Med. 2023 Aug 3;18(1):96. doi: 10.1186/s13020-023-00797-7. Chin Med. 2023. PMID: 37537605 Free PMC article. Review.
-
Atractylodin modulates ASAH3L to improve galactose metabolism and inflammation to alleviate acute lung injury.iScience. 2024 Aug 31;27(10):110751. doi: 10.1016/j.isci.2024.110751. eCollection 2024 Oct 18. iScience. 2024. PMID: 39351199 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 110-S-0023-E/iEGG and Animal Biotechnology Center from the Feature Areas Research Center Program within the framework of the Higher Education Sprout Project by the Taiwan Ministry of Education
- DMR-109-011/China Medical University Hospital
- TTMHH-NCHULS110001/National Chung-Hsing University/Tungs' Taichung MetroHarbor Hospital
- TTMHH-R1100048/Tungs' Taichung MetroHarbor Hospital
LinkOut - more resources
Full Text Sources
Research Materials

