Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review
- PMID: 34715347
- PMCID: PMC8548286
- DOI: 10.1016/j.cmi.2021.10.005
Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review
Abstract
Background: Vaccines are critical cost-effective tools to control the coronavirus disease 2019 (COVID-19) pandemic. However, the emergence of variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may threaten the global impact of mass vaccination campaigns.
Aims: The objective of this study was to provide an up-to-date comparative analysis of the characteristics, adverse events, efficacy, effectiveness and impact of the variants of concern for 19 COVID-19 vaccines.
Sources: References for this review were identified through searches of PubMed, Google Scholar, BioRxiv, MedRxiv, regulatory drug agencies and pharmaceutical companies' websites up to 22nd September 2021.
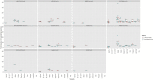
Content: Overall, all COVID-19 vaccines had a high efficacy against the original strain and the variants of concern, and were well tolerated. BNT162b2, mRNA-1273 and Sputnik V after two doses had the highest efficacy (>90%) in preventing symptomatic cases in phase III trials. mRNA vaccines, AZD1222, and CoronaVac were effective in preventing symptomatic COVID-19 and severe infections against Alpha, Beta, Gamma or Delta variants. Regarding observational real-life data, full immunization with mRNA vaccines and AZD1222 seems to effectively prevent SARS-CoV-2 infection against the original strain and Alpha and Beta variants but with reduced effectiveness against the Delta strain. A decline in infection protection was observed at 6 months for BNT162b2 and AZD1222. Serious adverse event rates were rare for mRNA vaccines-anaphylaxis 2.5-4.7 cases per million doses, myocarditis 3.5 cases per million doses-and were similarly rare for all other vaccines. Prices for the different vaccines varied from $2.15 to $29.75 per dose.
Implications: All vaccines appear to be safe and effective tools to prevent severe COVID-19, hospitalization, and death against all variants of concern, but the quality of evidence greatly varies depending on the vaccines considered. Questions remain regarding a booster dose and waning immunity, the duration of immunity, and heterologous vaccination. The benefits of COVID-19 vaccination outweigh the risks, despite rare serious adverse effects.
Keywords: COVID-19; Coronavirus; Delta; Efficacy; Review; SARS-CoV-2; Seroneutralization; Vaccines; Variants.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article. Review.
-
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar.N Engl J Med. 2022 May 12;386(19):1804-1816. doi: 10.1056/NEJMoa2200797. Epub 2022 Mar 9. N Engl J Med. 2022. PMID: 35263534 Free PMC article.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Covid-19 vaccines and variants of concern: A review.Rev Med Virol. 2022 Jul;32(4):e2313. doi: 10.1002/rmv.2313. Epub 2021 Nov 9. Rev Med Virol. 2022. PMID: 34755408 Free PMC article. Review.
Cited by
-
Safety, efficacy and immunogenicity of aerosolized Ad5-nCoV COVID-19 vaccine in a non-inferiority randomized controlled trial.NPJ Vaccines. 2024 Oct 31;9(1):209. doi: 10.1038/s41541-024-01003-x. NPJ Vaccines. 2024. PMID: 39482336 Free PMC article.
-
Exploiting reverse vaccinology approach for the design of a multiepitope subunit vaccine against the major SARS-CoV-2 variants.Comput Biol Chem. 2022 Dec;101:107754. doi: 10.1016/j.compbiolchem.2022.107754. Epub 2022 Aug 18. Comput Biol Chem. 2022. PMID: 36037724 Free PMC article.
-
Combining mRNA with PBS and calcium ions improves the efficiency of the transfection of mRNA into tumors.Mol Ther Nucleic Acids. 2024 Jul 17;35(3):102273. doi: 10.1016/j.omtn.2024.102273. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39184192 Free PMC article.
-
A pan-SARS-CoV-2-specific soluble angiotensin-converting enzyme 2-albumin fusion engineered for enhanced plasma half-life and needle-free mucosal delivery.PNAS Nexus. 2023 Nov 28;2(12):pgad403. doi: 10.1093/pnasnexus/pgad403. eCollection 2023 Dec. PNAS Nexus. 2023. PMID: 38077689 Free PMC article.
-
Current development of severe acute respiratory syndrome coronavirus 2 neutralizing antibodies (Review).Mol Med Rep. 2024 Aug;30(2):148. doi: 10.3892/mmr.2024.13272. Epub 2024 Jun 28. Mol Med Rep. 2024. PMID: 38940338 Free PMC article. Review.
References
-
- World Health Organization Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... [Internet]. [cité 30 mai 2021]
-
- Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021:1–17. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

