DPP inhibition alters the CXCR3 axis and enhances NK and CD8+ T cell infiltration to improve anti-PD1 efficacy in murine models of pancreatic ductal adenocarcinoma
- PMID: 34737215
- PMCID: PMC8578994
- DOI: 10.1136/jitc-2021-002837
DPP inhibition alters the CXCR3 axis and enhances NK and CD8+ T cell infiltration to improve anti-PD1 efficacy in murine models of pancreatic ductal adenocarcinoma
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is projected to be the second leading cause of cancer death in the USA by 2030. Immune checkpoint inhibitors fail to control most PDAC tumors because of PDAC's extensive immunosuppressive microenvironment and poor immune infiltration, a phenotype also seen in other non-inflamed (ie, 'cold') tumors. Identifying novel ways to enhance immunotherapy efficacy in PDAC is critical. Dipeptidyl peptidase (DPP) inhibition can enhance immunotherapy efficacy in other cancer types; however, the impact of DPP inhibition on PDAC tumors remains unexplored.
Methods: We examined the effects of an oral small molecule DPP inhibitor (BXCL701) on PDAC tumor growth using mT3-2D and Pan02 subcutaneous syngeneic murine models in C57BL/6 mice. We explored the effects of DPP inhibition on the tumor immune landscape using RNAseq, immunohistochemistry, cytokine evaluation and flow cytometry. We then tested if BXCL701 enhanced anti-programmed cell death protein 1 (anti-PD1) efficacy and performed immune cell depletion and rechallenged studies to explore the relevance of cytotoxic immune cells to combination treatment efficacy.
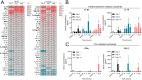
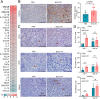
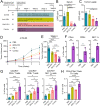
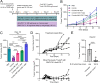
Results: In both murine models of PDAC, DPP inhibition enhanced NK and T cell immune infiltration and reduced tumor growth. DPP inhibition also enhanced the efficacy of anti-PD1. The efficacy of dual anti-PD1 and BXCL701 therapy was dependent on both CD8+ T cells and NK cells. Mice treated with this combination therapy developed antitumor immune memory that cleared some tumors after re-exposure. Lastly, we used The Cancer Genome Atlas (TCGA) to demonstrate that increased NK cell content, but not T cell content, in human PDAC tumors is correlated with longer overall survival. We propose that broad DPP inhibition enhances antitumor immune response via two mechanisms: (1) DPP4 inhibition increases tumor content of CXCL9/10, which recruits CXCR3+ NK and T cells, and (2) DPP8/9 inhibition activates the inflammasome, resulting in proinflammatory cytokine release and Th1 response, further enhancing the CXCL9/10-CXCR3 axis.
Conclusions: These findings show that DPP inhibition with BXCL701 represents a pharmacologic strategy to increase the tumor microenvironment immune cell content to improve anti-PD1 efficacy in PDAC, suggesting BXCL701 can enhance immunotherapy efficacy in 'cold' tumor types. These findings also highlight the potential importance of NK cells along with T cells in regulating PDAC tumor growth.
Keywords: combination; drug evaluation; drug therapy; immunotherapy; preclinical; programmed cell death 1 receptor; th1-th2 balance.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LMW received research funding from BioXcel Therapeutics.
Figures


Similar articles
-
SIN3B Loss Heats up Cold Tumor Microenvironment to Boost Immunotherapy in Pancreatic Cancer.Adv Sci (Weinh). 2024 Nov;11(43):e2402244. doi: 10.1002/advs.202402244. Epub 2024 Sep 24. Adv Sci (Weinh). 2024. PMID: 39316363 Free PMC article.
-
Unraveling the impact of sialic acids on the immune landscape and immunotherapy efficacy in pancreatic cancer.J Immunother Cancer. 2023 Nov;11(11):e007805. doi: 10.1136/jitc-2023-007805. J Immunother Cancer. 2023. PMID: 37940346 Free PMC article.
-
Intratumoral injection of TLR9 agonist promotes an immunopermissive microenvironment transition and causes cooperative antitumor activity in combination with anti-PD1 in pancreatic cancer.J Immunother Cancer. 2021 Sep;9(9):e002876. doi: 10.1136/jitc-2021-002876. J Immunother Cancer. 2021. PMID: 34479922 Free PMC article.
-
Pancreatic ductal adenocarcinoma microenvironment: Soluble factors and cancer associated fibroblasts as modulators of NK cell functions.Immunol Lett. 2024 Oct;269:106898. doi: 10.1016/j.imlet.2024.106898. Epub 2024 Jul 15. Immunol Lett. 2024. PMID: 39019404 Review.
-
Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options.Front Immunol. 2018 Aug 15;9:1878. doi: 10.3389/fimmu.2018.01878. eCollection 2018. Front Immunol. 2018. PMID: 30158932 Free PMC article. Review.
Cited by
-
The role of CXCR3 and its ligands in cancer.Front Oncol. 2022 Nov 21;12:1022688. doi: 10.3389/fonc.2022.1022688. eCollection 2022. Front Oncol. 2022. PMID: 36479091 Free PMC article. Review.
-
Clinical trial-identified inflammatory biomarkers in breast and pancreatic cancers.Front Endocrinol (Lausanne). 2023 Apr 27;14:1106520. doi: 10.3389/fendo.2023.1106520. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37181043 Free PMC article. Review.
-
Identification of gene signatures related to hypoxia and angiogenesis in pancreatic cancer to aid immunotherapy and prognosis.Front Oncol. 2023 Mar 30;13:1119763. doi: 10.3389/fonc.2023.1119763. eCollection 2023. Front Oncol. 2023. PMID: 37064125 Free PMC article.
-
Does DPP-IV Inhibition Offer New Avenues for Therapeutic Intervention in Malignant Disease?Cancers (Basel). 2022 Apr 21;14(9):2072. doi: 10.3390/cancers14092072. Cancers (Basel). 2022. PMID: 35565202 Free PMC article. Review.
-
NK cells and solid tumors: therapeutic potential and persisting obstacles.Mol Cancer. 2022 Nov 1;21(1):206. doi: 10.1186/s12943-022-01672-z. Mol Cancer. 2022. PMID: 36319998 Free PMC article. Review.
References
-
- Society AC . Cancer facts & figures 20221 [online]. Available: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
