Pivotal Evaluation of an Artificial Intelligence System for Autonomous Detection of Referrable and Vision-Threatening Diabetic Retinopathy
- PMID: 34779843
- PMCID: PMC8593763
- DOI: 10.1001/jamanetworkopen.2021.34254
Pivotal Evaluation of an Artificial Intelligence System for Autonomous Detection of Referrable and Vision-Threatening Diabetic Retinopathy
Erratum in
-
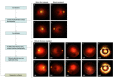
Error in Figure 2.JAMA Netw Open. 2021 Dec 1;4(12):e2144317. doi: 10.1001/jamanetworkopen.2021.44317. JAMA Netw Open. 2021. PMID: 34935926 Free PMC article. No abstract available.
Abstract
Importance: Diabetic retinopathy (DR) is a leading cause of blindness in adults worldwide. Early detection and intervention can prevent blindness; however, many patients do not receive their recommended annual diabetic eye examinations, primarily owing to limited access.
Objective: To evaluate the safety and accuracy of an artificial intelligence (AI) system (the EyeArt Automated DR Detection System, version 2.1.0) in detecting both more-than-mild diabetic retinopathy (mtmDR) and vision-threatening diabetic retinopathy (vtDR).
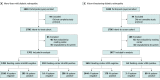
Design, setting, and participants: A prospective multicenter cross-sectional diagnostic study was preregistered (NCT03112005) and conducted from April 17, 2017, to May 30, 2018. A total of 942 individuals aged 18 years or older who had diabetes gave consent to participate at 15 primary care and eye care facilities. Data analysis was performed from February 14 to July 10, 2019.
Interventions: Retinal imaging for the autonomous AI system and Early Treatment Diabetic Retinopathy Study (ETDRS) reference standard determination.
Main outcomes and measures: Primary outcome measures included the sensitivity and specificity of the AI system in identifying participants' eyes with mtmDR and/or vtDR by 2-field undilated fundus photography vs a rigorous clinical reference standard comprising reading center grading of 4 wide-field dilated images using the ETDRS severity scale. Secondary outcome measures included the evaluation of imageability, dilated-if-needed analysis, enrichment correction analysis, worst-case imputation, and safety outcomes.
Results: Of 942 consenting individuals, 893 patients (1786 eyes) met the inclusion criteria and completed the study protocol. The population included 449 men (50.3%). Mean (SD) participant age was 53.9 (15.2) years (median, 56; range, 18-88 years), 655 were White (73.3%), and 206 had type 1 diabetes (23.1%). Sensitivity and specificity of the AI system were high in detecting mtmDR (sensitivity: 95.5%; 95% CI, 92.4%-98.5% and specificity: 85.0%; 95% CI, 82.6%-87.4%) and vtDR (sensitivity: 95.1%; 95% CI, 90.1%-100% and specificity: 89.0%; 95% CI, 87.0%-91.1%) without dilation. Imageability was high without dilation, with the AI system able to grade 87.4% (95% CI, 85.2%-89.6%) of the eyes with reading center grades. When eyes with ungradable results were dilated per the protocol, the imageability improved to 97.4% (95% CI, 96.4%-98.5%), with the sensitivity and specificity being similar. After correcting for enrichment, the mtmDR specificity increased to 87.8% (95% CI, 86.3%-89.5%) and the sensitivity remained similar; for vtDR, both sensitivity (97.0%; 95% CI, 91.2%-100%) and specificity (90.1%; 95% CI, 89.4%-91.5%) improved.
Conclusions and relevance: This prospective multicenter cross-sectional diagnostic study noted safety and accuracy with use of the EyeArt Automated DR Detection System in detecting both mtmDR and, for the first time, vtDR, without physician assistance. These findings suggest that improved access to accurate, reliable diabetic eye examinations may increase adherence to recommended annual screenings and allow for accelerated referral of patients identified as having vtDR.
Conflict of interest statement
Figures
Similar articles
-
Artificial Intelligence Detection of Diabetic Retinopathy: Subgroup Comparison of the EyeArt System with Ophthalmologists' Dilated Examinations.Ophthalmol Sci. 2022 Sep 30;3(1):100228. doi: 10.1016/j.xops.2022.100228. eCollection 2023 Mar. Ophthalmol Sci. 2022. PMID: 36345378 Free PMC article.
-
Automated Identification of Different Severity Levels of Diabetic Retinopathy Using a Handheld Fundus Camera and Single-Image Protocol.Ophthalmol Sci. 2024 Feb 7;4(4):100481. doi: 10.1016/j.xops.2024.100481. eCollection 2024 Jul-Aug. Ophthalmol Sci. 2024. PMID: 38694494 Free PMC article.
-
Evaluation of an Artificial Intelligence System for the Detection of Diabetic Retinopathy in Chinese Community Healthcare Centers.Front Med (Lausanne). 2022 Apr 11;9:883462. doi: 10.3389/fmed.2022.883462. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35479949 Free PMC article.
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD008081. doi: 10.1002/14651858.CD008081.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jan 07;1:CD008081. doi: 10.1002/14651858.CD008081.pub3 PMID: 21735421 Updated. Review.
-
Use of Smartphones to Detect Diabetic Retinopathy: Scoping Review and Meta-Analysis of Diagnostic Test Accuracy Studies.J Med Internet Res. 2020 May 15;22(5):e16658. doi: 10.2196/16658. J Med Internet Res. 2020. PMID: 32347810 Free PMC article. Review.
Cited by
-
Ophthalmic imaging in diabetic retinopathy: A review.Clin Exp Ophthalmol. 2022 Dec;50(9):1082-1096. doi: 10.1111/ceo.14170. Epub 2022 Sep 25. Clin Exp Ophthalmol. 2022. PMID: 36102668 Free PMC article. Review.
-
Artificial intelligence-driven transformations in diabetes care: a comprehensive literature review.Ann Med Surg (Lond). 2024 Jul 23;86(9):5334-5342. doi: 10.1097/MS9.0000000000002369. eCollection 2024 Sep. Ann Med Surg (Lond). 2024. PMID: 39238969 Free PMC article. Review.
-
Diabetes and artificial intelligence beyond the closed loop: a review of the landscape, promise and challenges.Diabetologia. 2024 Feb;67(2):223-235. doi: 10.1007/s00125-023-06038-8. Epub 2023 Nov 18. Diabetologia. 2024. PMID: 37979006 Free PMC article. Review.
-
Development and Validation of a Diabetic Retinopathy Risk Stratification Algorithm.Diabetes Care. 2023 May 1;46(5):1068-1075. doi: 10.2337/dc22-1168. Diabetes Care. 2023. PMID: 36930723 Free PMC article.
-
The gut-retina axis: a new perspective in the prevention and treatment of diabetic retinopathy.Front Endocrinol (Lausanne). 2023 Jul 4;14:1205846. doi: 10.3389/fendo.2023.1205846. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37469982 Free PMC article. Review.
References
-
- International Federation on Ageing . International Agency for the Prevention of Blindness; International Diabetes Federation. The Diabetic Retinopathy Barometer Report Global Findings. Accessed October 1, 2021. https://www.iapb.org/wp-content/uploads/DR-Global-Report-1.pdf
-
- Centers for Disease Control and Prevention . Diabetes Report Card 2017. US Dept of Health and Human Services; 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

