Cerium(IV) and Iron(III) Oxides Nanoparticles Based Voltammetric Sensor for the Sensitive and Selective Determination of Lipoic Acid
- PMID: 34833711
- PMCID: PMC8621773
- DOI: 10.3390/s21227639
Cerium(IV) and Iron(III) Oxides Nanoparticles Based Voltammetric Sensor for the Sensitive and Selective Determination of Lipoic Acid
Abstract
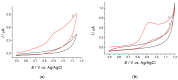
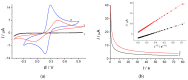
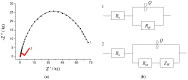
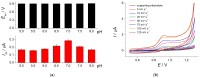
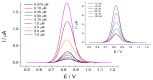
A novel voltammetric sensor based on CeO2·Fe2O3 nanoparticles (NPs) has been developed for the determination of lipoic acid, playing an essential role in aerobic metabolism in the living organism. Sensor surface modification provides a 5.6-fold increase of the lipoic acid oxidation currents and a 20 mV anodic shift of the oxidation potential. The best voltammetric parameters have been obtained for the 0.5 mg mL-1 dispersion of CeO2·Fe2O3 NPs. Scanning electron microscopy (SEM) confirms the presence of spherical NPs of 25-60 nm, and their aggregates evenly distributed on the electrode surface and formed porous coverage. This leads to the 4.4-fold increase of the effective surface area vs. bare glassy carbon electrode (GCE). The sensor shows a significantly higher electron transfer rate. Electrooxidation of lipoic acid on CeO2·Fe2O3 NPs modified GCE is an irreversible diffusion-controlled pH-independent process occurring with the participation of two electrons. The sensor gives a linear response to lipoic acid in the ranges of 0.075-7.5 and 7.5-100 μM with the detection limit of 0.053 μM. The sensor is selective towards lipoic acid in the presence of inorganic ions, ascorbic acid, saccharides, and other S-containing compounds. The sensor developed has been tested on the pharmaceutical dosage forms of lipoic acid.
Keywords: amperometric sensors; electrooxidation; lipoic acid; metal oxide nanoparticles; pharmaceutical analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


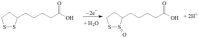
Similar articles
-
Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF.Sensors (Basel). 2024 Jan 31;24(3):930. doi: 10.3390/s24030930. Sensors (Basel). 2024. PMID: 38339646 Free PMC article.
-
Ultrasound treated cerium oxide/tin oxide (CeO2/SnO2) nanocatalyst: A feasible approach and enhanced electrode material for sensing of anti-inflammatory drug 5-aminosalicylic acid in biological samples.Anal Chim Acta. 2020 Feb 1;1096:76-88. doi: 10.1016/j.aca.2019.10.059. Epub 2019 Oct 31. Anal Chim Acta. 2020. PMID: 31883594
-
Amperometric sensing of hydrazine in environmental and biological samples by using CeO2-encapsulated gold nanoparticles on reduced graphene oxide.Mikrochim Acta. 2019 Jan 4;186(1):46. doi: 10.1007/s00604-018-3144-4. Mikrochim Acta. 2019. PMID: 30610467
-
Voltammetric Sensor Based on SeO2 Nanoparticles and Surfactants for Indigo Carmine Determination.Sensors (Basel). 2022 Apr 22;22(9):3224. doi: 10.3390/s22093224. Sensors (Basel). 2022. PMID: 35590915 Free PMC article.
-
Recent Advances in Electrochemical Sensors for Sulfur-Containing Antioxidants.Micromachines (Basel). 2023 Jul 18;14(7):1440. doi: 10.3390/mi14071440. Micromachines (Basel). 2023. PMID: 37512751 Free PMC article. Review.
Cited by
-
A Molecularly Imprinted Polypyrrole/GO@Fe3O4 Nanocomposite Modified Impedimetric Sensor for the Routine Monitoring of Lysozyme.Biosensors (Basel). 2022 Sep 5;12(9):727. doi: 10.3390/bios12090727. Biosensors (Basel). 2022. PMID: 36140112 Free PMC article.
-
Selective Voltammetric Sensor for the Simultaneous Quantification of Tartrazine and Brilliant Blue FCF.Sensors (Basel). 2023 Jan 17;23(3):1094. doi: 10.3390/s23031094. Sensors (Basel). 2023. PMID: 36772133 Free PMC article.
-
Novel Iron Oxide Nanoparticle-Fortified Carbon Paste Electrode for the Sensitive Voltammetric Determination of Atomoxetine.ACS Omega. 2023 May 18;8(21):19006-19015. doi: 10.1021/acsomega.3c01726. eCollection 2023 May 30. ACS Omega. 2023. PMID: 37273581 Free PMC article.
-
Synthesis of ultrasmall cerium oxide nanoparticles in deep eutectic solvent and their application in an electrochemical sensor to detect dopamine in biological fluid.Mikrochim Acta. 2024 Jun 27;191(7):425. doi: 10.1007/s00604-024-06480-4. Mikrochim Acta. 2024. PMID: 38926184
-
Voltammetric Sensor Based on the Combination of Tin and Cerium Dioxide Nanoparticles with Surfactants for Quantification of Sunset Yellow FCF.Sensors (Basel). 2024 Jan 31;24(3):930. doi: 10.3390/s24030930. Sensors (Basel). 2024. PMID: 38339646 Free PMC article.
References
-
- Marin M., Lete C., Manolescu B.N., Lupu S. Electrochemical determination of α-lipoic acid in human serum at platinum electrode. J. Electroanal. Chem. 2014;729:128–134. doi: 10.1016/j.jelechem.2014.07.024. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

