Collagen 1a1 Expression by Airway Macrophages Increases In Fibrotic ILDs and Is Associated With FVC Decline and Increased Mortality
- PMID: 34867934
- PMCID: PMC8635798
- DOI: 10.3389/fimmu.2021.645548
Collagen 1a1 Expression by Airway Macrophages Increases In Fibrotic ILDs and Is Associated With FVC Decline and Increased Mortality
Abstract
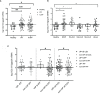
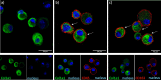
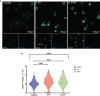
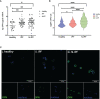
Within the Interstitial Lung Diseases (ILD), patients with idiopathic pulmonary fibrosis (IPF) and a subset of those with non-IPF fibrotic ILD have a distinct clinical phenotype of progression despite management. This group of patients has been collectively termed the progressive fibrotic phenotype (PFP). Their early recognition may facilitate access to antifibrotic therapies to prevent or slow progression. Macrophages/monocytes within the lung orchestrate the progression and maintenance of fibrosis. A novel role for monocyte-derived macrophages during tissue damage and wound healing is the expression of collagens. We examined Collagen 1a1 expression in airway macrophages from ILD patients at diagnosis. COL1A1 mRNA levels from BAL cells were elevated in IPF and Non-IPF patients. The presence of a UIP pattern and a subsequent progressive phenotype were significantly associated with the higher BAL COL1A1 levels. In Non-IPF patients, higher COL1A1 levels were associated with a more than twofold increase in mortality. The intracellular localisation of COL1A1 in airway macrophages was demonstrated by confocal microscopy in CD45 and CD163 co-staining assays. Additionally, airway macrophages co-expressed COL1A1 with the profibrotic SPP1 gene product osteopontin. The levels of SPP1 mRNA and OPN in the BAL were significantly higher in IPF and Non-IPF patients relative to healthy. Our results suggest that profibrotic airway macrophages are increased in the BAL of patients with IPF and other ILDs and co-express COL1A1 and OPN. Importantly, COL1A1 expression by pro-fibrotic airway macrophages could be a marker of disease progression and poor survival in ILDs.
Keywords: IPF; NSIP; PF-ILD; RA-ILD; SPP1; airway macrophages; collagen 1A1; osteopontin.
Copyright © 2021 Tsitoura, Trachalaki, Vasarmidi, Mastrodemou, Margaritopoulos, Kokosi, Fanidis, Galaris, Aidinis, Renzoni, Tzanakis, Wells and Antoniou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
CCL18 in serum, BAL fluid and alveolar macrophage culture supernatant in interstitial lung diseases.Respir Med. 2013 Sep;107(9):1444-52. doi: 10.1016/j.rmed.2013.06.004. Epub 2013 Jul 5. Respir Med. 2013. PMID: 23831213
-
Essential role of osteopontin in smoking-related interstitial lung diseases.Am J Pathol. 2009 May;174(5):1683-91. doi: 10.2353/ajpath.2009.080689. Epub 2009 Apr 9. Am J Pathol. 2009. PMID: 19359522 Free PMC article.
-
Nintedanib: A Review in Fibrotic Interstitial Lung Diseases.Drugs. 2021 Apr;81(5):575-586. doi: 10.1007/s40265-021-01487-0. Epub 2021 Mar 25. Drugs. 2021. PMID: 33765296 Free PMC article. Review.
-
Association between Pepsin in Bronchoalveolar Lavage Fluid and Prognosis of Chronic Fibrosing Interstitial Lung Disease.Tohoku J Exp Med. 2018 Nov;246(3):147-153. doi: 10.1620/tjem.246.147. Tohoku J Exp Med. 2018. PMID: 30405002
-
A cohort study of Danish patients with interstitial lung diseases: burden, severity, treatment and survival.Dan Med J. 2015 Apr;62(4):B5069. Dan Med J. 2015. PMID: 25872544 Review.
Cited by
-
Influence of the At-Arrival Host Transcriptome on Bovine Respiratory Disease Incidence during Backgrounding.Vet Sci. 2023 Mar 10;10(3):211. doi: 10.3390/vetsci10030211. Vet Sci. 2023. PMID: 36977250 Free PMC article.
-
Cellular and molecular mechanisms of fibrosis and resolution in bleomycin-induced pulmonary fibrosis mouse model revealed by spatial transcriptome analysis.Heliyon. 2023 Nov 20;9(12):e22461. doi: 10.1016/j.heliyon.2023.e22461. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125541 Free PMC article.
-
Effects of Anti-Fibrotic Drugs on Transcriptome of Peripheral Blood Mononuclear Cells in Idiopathic Pulmonary Fibrosis.Int J Mol Sci. 2024 Mar 28;25(7):3750. doi: 10.3390/ijms25073750. Int J Mol Sci. 2024. PMID: 38612561 Free PMC article.
-
A genomic perspective of the aging human and mouse lung with a focus on immune response and cellular senescence.Immun Ageing. 2023 Nov 6;20(1):58. doi: 10.1186/s12979-023-00373-5. Immun Ageing. 2023. PMID: 37932771 Free PMC article.
-
Multi-omics integration reveals a nonlinear signature that precedes progression of lung fibrosis.Clin Transl Immunology. 2024 Jan 24;13(1):e1485. doi: 10.1002/cti2.1485. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38269243 Free PMC article.
References
-
- Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. . An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med (2013) 188(6):733–48. doi: 10.1164/rccm.201308-1483ST - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

