Mechanisms of FA-Phagy, a New Form of Selective Autophagy/Organellophagy
- PMID: 34950664
- PMCID: PMC8689057
- DOI: 10.3389/fcell.2021.799123
Mechanisms of FA-Phagy, a New Form of Selective Autophagy/Organellophagy
Abstract
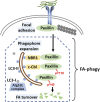
Focal adhesions (FAs) are adhesive organelles that attach cells to the extracellular matrix and can mediate various biological functions in response to different environmental cues. Reduced FAs are often associated with enhanced cell migration and cancer metastasis. In addition, because FAs are essential for preserving vascular integrity, the loss of FAs leads to hemorrhages and is frequently observed in many vascular diseases such as intracranial aneurysms. For these reasons, FAs are an attractive therapeutic _target for treating cancer or vascular diseases, two leading causes of death world-wide. FAs are controlled by both their formation and turnover. In comparison to the large body of literature detailing FA formation, the mechanisms of FA turnover are poorly understood. Recently, autophagy has emerged as a major mechanism to degrade FAs and stabilizing FAs by inhibiting autophagy has a beneficial effect on breast cancer metastasis, suggesting autophagy-mediated FA turnover is a promising drug _target. Intriguingly, autophagy-mediated FA turnover is a selective process and the cargo receptors for recognizing FAs in this process are context-dependent, which ensures the degradation of specific cargo. This paper mainly reviews the cargo recognition mechanisms of FA-phagy (selective autophagy-mediated FA turnover) and its disease relevance. We seek to outline some new points of understanding that will facilitate further study of FA-phagy and precise therapeutic strategies for related diseases associated with aberrant FA functions.
Keywords: autophagy; cancer; cargo receptor; focal adhesion; intracranial aneurysm; organellophagy; vascular integrity.
Copyright © 2021 Lu, Linares, Xu and Rui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
THSD1 Suppresses Autophagy-Mediated Focal Adhesion Turnover by Modulating the FAK-Beclin 1 Pathway.Int J Mol Sci. 2024 Feb 10;25(4):2139. doi: 10.3390/ijms25042139. Int J Mol Sci. 2024. PMID: 38396816 Free PMC article.
-
NBR1 enables autophagy-dependent focal adhesion turnover.J Cell Biol. 2016 Feb 29;212(5):577-90. doi: 10.1083/jcb.201503075. Epub 2016 Feb 22. J Cell Biol. 2016. PMID: 26903539 Free PMC article.
-
NBR1-dependent selective autophagy is required for efficient cell-matrix adhesion site disassembly.Autophagy. 2016 Oct 2;12(10):1958-1959. doi: 10.1080/15548627.2016.1212789. Epub 2016 Aug 2. Autophagy. 2016. PMID: 27484104 Free PMC article.
-
Autophagy in adhesion and migration.J Cell Sci. 2016 Oct 15;129(20):3685-3693. doi: 10.1242/jcs.188490. Epub 2016 Sep 26. J Cell Sci. 2016. PMID: 27672021 Free PMC article. Review.
-
Assembly and mechanosensory function of focal adhesions: experiments and models.Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review.
Cited by
-
A novel focal adhesion-related risk model predicts prognosis of bladder cancer -- a bioinformatic study based on TCGA and GEO database.BMC Cancer. 2022 Nov 10;22(1):1158. doi: 10.1186/s12885-022-10264-5. BMC Cancer. 2022. PMID: 36357874 Free PMC article.
-
_targeting selective autophagy and beyond: From underlying mechanisms to potential therapies.J Adv Res. 2024 Nov;65:297-327. doi: 10.1016/j.jare.2024.05.009. Epub 2024 May 14. J Adv Res. 2024. PMID: 38750694 Free PMC article. Review.
-
THSD1 Suppresses Autophagy-Mediated Focal Adhesion Turnover by Modulating the FAK-Beclin 1 Pathway.Int J Mol Sci. 2024 Feb 10;25(4):2139. doi: 10.3390/ijms25042139. Int J Mol Sci. 2024. PMID: 38396816 Free PMC article.
-
Starvation-inactivated MTOR triggers cell migration via a ULK1-SH3PXD2A/TKS5-MMP14 pathway in ovarian carcinoma.Autophagy. 2023 Dec;19(12):3151-3168. doi: 10.1080/15548627.2023.2239633. Epub 2023 Jul 28. Autophagy. 2023. PMID: 37505094 Free PMC article.
-
Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: insights into adhesomes, mechanical forces, and signaling pathways.Front Cell Dev Biol. 2023 Nov 30;11:1221306. doi: 10.3389/fcell.2023.1221306. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099295 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

