A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia)
- PMID: 35164083
- PMCID: PMC8840237
- DOI: 10.3390/molecules27030820
A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia)
Abstract
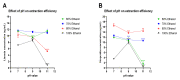
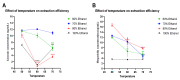
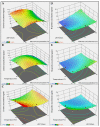
Green extraction is aimed at reducing energy consumption by using renewable plant sources and environmentally friendly bio-solvents. Lime (Citrus aurantifolia) is a rich source of flavonoids (e.g., hesperidin) and limonoids (e.g., limonin). Manufacturing of lime products (e.g., lime juice) yields a considerable amount of lime peel as food waste that should be comprehensively exploited. The aim of this study was to develop a green and simple extraction method to acquire the highest yield of both limonin and hesperidin from the lime peel. The study method included ethanolic-aqueous extraction and variable factors, i.e., ethanol concentrations, pH values of solvent, and extraction temperature. The response surface methodology was used to optimize extraction conditions. The concentrations of limonin and hesperidin were determined by using UHPLC-MS/MS. Results showed that the yields of limonin and hesperidin significantly depended on ethanol concentrations and extraction temperature, while pH value had the least effect. The optimal extraction condition with the highest amounts of limonin and hesperidin was 80% ethanol at pH 7, 50 °C, which yields 2.072 and 3.353 mg/g of limonin and hesperidin, respectively. This study illustrates a green extraction process using food waste, e.g., lime peel, as an energy-saving source and ethanol as a bio-solvent to achieve the highest amount of double bioactive compounds.
Keywords: LC-MS/MS; citrus; food waste; green extraction; hesperidin; lime; limonin.
Conflict of interest statement
The authors declare no conflict of interest or personal relationships with other people or organizations that can inappropriately influence our work.
Figures

Similar articles
-
Metabolomic Analysis of Phytochemical Compounds from Ethanolic Extract of Lime (Citrus aurantifolia) Peel and Its Anti-Cancer Effects against Human Hepatocellular Carcinoma Cells.Molecules. 2023 Mar 26;28(7):2965. doi: 10.3390/molecules28072965. Molecules. 2023. PMID: 37049726 Free PMC article.
-
Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development.Foods. 2023 May 17;12(10):2036. doi: 10.3390/foods12102036. Foods. 2023. PMID: 37238855 Free PMC article. Review.
-
Recovery and purification of limonin from pummelo [Citrus grandis] peel using water extraction, ammonium sulfate precipitation and resin adsorption.J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Aug 15;1060:150-157. doi: 10.1016/j.jchromb.2017.05.036. Epub 2017 Jun 3. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 28622618
-
Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel.Ultrason Sonochem. 2008 Mar;15(3):227-32. doi: 10.1016/j.ultsonch.2007.03.006. Epub 2007 Apr 2. Ultrason Sonochem. 2008. PMID: 17584518
-
Hesperidin: A Review on Extraction Methods, Stability and Biological Activities.Nutrients. 2022 Jun 9;14(12):2387. doi: 10.3390/nu14122387. Nutrients. 2022. PMID: 35745117 Free PMC article. Review.
Cited by
-
Optimization of Phytochemical-Rich Citrus maxima Albedo Extract Using Response Surface Methodology.Molecules. 2023 May 16;28(10):4121. doi: 10.3390/molecules28104121. Molecules. 2023. PMID: 37241861 Free PMC article.
-
Metabolomic Analysis of Phytochemical Compounds from Ethanolic Extract of Lime (Citrus aurantifolia) Peel and Its Anti-Cancer Effects against Human Hepatocellular Carcinoma Cells.Molecules. 2023 Mar 26;28(7):2965. doi: 10.3390/molecules28072965. Molecules. 2023. PMID: 37049726 Free PMC article.
-
Influence of Different Extraction Methods on the Changes in Bioactive Compound Composition and Antioxidant Properties of Solid-State Fermented Coffee Husk Extracts.ScientificWorldJournal. 2023 Sep 21;2023:6698056. doi: 10.1155/2023/6698056. eCollection 2023. ScientificWorldJournal. 2023. PMID: 37780638 Free PMC article.
-
Therapeutic efficacy of Citrus aurantifolia (lime) juice in experimental Eimeria tenella-infected broiler chickens.Trop Anim Health Prod. 2023 Dec 11;56(1):8. doi: 10.1007/s11250-023-03840-9. Trop Anim Health Prod. 2023. PMID: 38072881
-
Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development.Foods. 2023 May 17;12(10):2036. doi: 10.3390/foods12102036. Foods. 2023. PMID: 37238855 Free PMC article. Review.
References
-
- Shahbandeh M. Leading Fresh Lemon and Lime Producers Worldwide in 2020/2021. [(accessed on 18 November 2021)]. Available online: https://www.statista.com/statistics/1045016/world-lemons-and-limes-major...
-
- Ledesma-Escobar C., Castro M. Towards a comprehensive exploitation of citrus. Trends Food Sci. Technol. 2014;39:63–74. doi: 10.1016/j.tifs.2014.07.002. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

