Glucagon-Like Peptide-1 Receptor Agonist Use in People Living with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Narrative Review of the Key Evidence with Practical Considerations
- PMID: 35175551
- PMCID: PMC8934828
- DOI: 10.1007/s13300-021-01198-5
Glucagon-Like Peptide-1 Receptor Agonist Use in People Living with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Narrative Review of the Key Evidence with Practical Considerations
Abstract
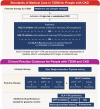
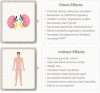
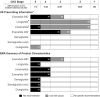
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are incretin-mimetic agents that are effective adjuncts in the treatment of diabetes. This class of medications is also associated with promoting weight loss and a low risk of hypoglycemia, and some have been shown to be associated with a significant reduction of major cardiovascular events. Mounting evidence suggests that GLP-1 RAs have benefits beyond reducing blood glucose that include improving kidney function in people living with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD), a common microvascular complication of T2DM. Several large clinical studies, the majority of which are cardiovascular outcome trials, indicate that GLP-1 RA therapy is safe and tolerable for people living with T2DM and compromised renal function, and also suggest that GLP-1 RAs may have renoprotective properties. Although evidence from clinical trials has shown GLP-1 RAs to be safe and efficacious in people living with T2DM and renal impairment, their use is uncommon in this patient population. With continuing developments in the field of GLP-1 RA therapy, it is important for physicians to understand the benefits and practical use of GLP-1 RAs, as well as the clinical evidence, in order to achieve positive patient outcomes. Here, we review evidence on GLP-1 RA use in people living with T2DM and CKD and summarize renal outcomes from clinical studies. We provide practical considerations for GLP-1 RA use to provide an added benefit to guide treatment in this high-risk patient population.
Keywords: Chronic kidney disease; Diabetic kidney disease; Glucagon-like peptide-1 receptor agonists; Renal impairment; Type 2 diabetes mellitus.
Plain language summary
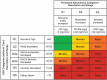
Type 2 diabetes mellitus (T2DM) is a common disorder characterized by insulin resistance and dysfunction of insulin-producing beta cells of the pancreas. People living with T2DM have an increased risk of developing complications, including chronic kidney disease (CKD), which itself is associated with increased mortality. Both the American Diabetes Association and Kidney Disease Improving Global Outcomes organization provide updated pharmacological recommendations for treating T2DM in people with CKD that include the use of sodium-glucose co-transporter-2 inhibitors (SGLT2i) or glucagon-like peptide-1 receptor agonists (GLP-1 RAs). GLP-1 RAs are effective and safe treatments for controlling blood sugar levels and reducing body weight, and evidence from large clinical trials also suggests that GLP-1 RAs may be renoprotective. Despite the benefits of GLP-1 RAs, they are not commonly prescribed in people living with T2DM and CKD. Healthcare practitioners need to be aware of the most recent information so that they can make informed decisions when selecting treatment options. The objective of this review is to summarize the main renal outcomes from clinical studies while providing practical guidance on the use of GLP-1 RAs.
© 2022. The Author(s).
Figures

Similar articles
-
Review of glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes mellitus in patients with chronic kidney disease and their renal effects.J Diabetes. 2019 Dec;11(12):938-948. doi: 10.1111/1753-0407.12969. Epub 2019 Aug 14. J Diabetes. 2019. PMID: 31318152 Free PMC article. Review.
-
SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline.BMJ. 2021 May 11;373:n1091. doi: 10.1136/bmj.n1091. BMJ. 2021. PMID: 33975892
-
Role of GLP-1 Receptor Agonist in Diabetic Cardio-renal Disorder: Recent Updates of Clinical and Pre-clinical Evidence.Curr Diabetes Rev. 2024;20(6):e090823219597. doi: 10.2174/1573399820666230809152148. Curr Diabetes Rev. 2024. PMID: 37559236 Review.
-
Real-World Effectiveness of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists (OW GLP-1RAs) in Comparison with Dipeptidyl Peptidase-4 Inhibitors (DPP-4is) for Glycemic Control and Weight Outcomes in Type 2 Diabetes Mellitus (RELATE).Clin Drug Investig. 2024 Apr;44(4):271-284. doi: 10.1007/s40261-024-01354-2. Epub 2024 Mar 20. Clin Drug Investig. 2024. PMID: 38507188 Free PMC article.
-
Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM).Cardiovasc Diabetol. 2023 Apr 1;22(1):79. doi: 10.1186/s12933-023-01798-4. Cardiovasc Diabetol. 2023. PMID: 37005640 Free PMC article. Review.
Cited by
-
Glucagon-like Peptide 1 Receptor Agonists in Cardio-Oncology: Pathophysiology of Cardiometabolic Outcomes in Cancer Patients.Int J Mol Sci. 2024 Oct 21;25(20):11299. doi: 10.3390/ijms252011299. Int J Mol Sci. 2024. PMID: 39457081 Free PMC article. Review.
-
Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database.Pharmaceuticals (Basel). 2024 Jan 23;17(2):147. doi: 10.3390/ph17020147. Pharmaceuticals (Basel). 2024. PMID: 38399362 Free PMC article.
-
Effect of GLP-1 receptor agonists on weight and cardiovascular outcomes: A review.Medicine (Baltimore). 2024 Nov 1;103(44):e40364. doi: 10.1097/MD.0000000000040364. Medicine (Baltimore). 2024. PMID: 39496023 Free PMC article. Review.
-
Safety and effectiveness of dulaglutide 0.75 mg in Japanese patients with type 2 diabetes in real-world clinical practice: 36 month post-marketing observational study.J Diabetes Investig. 2023 Feb;14(2):247-258. doi: 10.1111/jdi.13932. Epub 2022 Nov 11. J Diabetes Investig. 2023. PMID: 36367417 Free PMC article.
-
Comprehensive Cardiovascular and Renal Protection in Patients with Type 2 Diabetes.J Clin Med. 2023 Jun 8;12(12):3925. doi: 10.3390/jcm12123925. J Clin Med. 2023. PMID: 37373620 Free PMC article. Review.
References
-
- Kidney Disease: Improving Global Outcomes Diabetes Work Group (KDIGO) Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Inter, Suppl. 2020;2020(98):S1–S115. - PubMed
-
- American Diabetes Association 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S151–S167. - PubMed
-
- Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

