HR-LC-ESI-Orbitrap-MS-Based Metabolic Profiling Coupled with Chemometrics for the Discrimination of Different Echinops spinosus Organs and Evaluation of Their Antioxidant Activity
- PMID: 35326103
- PMCID: PMC8944760
- DOI: 10.3390/antiox11030453
HR-LC-ESI-Orbitrap-MS-Based Metabolic Profiling Coupled with Chemometrics for the Discrimination of Different Echinops spinosus Organs and Evaluation of Their Antioxidant Activity
Abstract
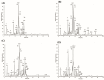
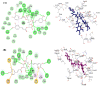
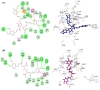
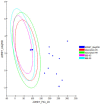
This study aimed to assess and correlate the phenolic content and the antioxidant activity of the methanol extracts of the stems, roots, flowers, and leaves of Echinops spinosus L. from north-eastern Algeria. Qualitative analysis was performed by high-resolution mass spectrometry (HR) LC-ESI-Orbitrap-MS and (HR) LC-ESI-Orbitrap-MS/MS). Forty-five compounds were identified in the methanol extracts; some are described for the first time in E. spinosus. _targeted phenolic compounds were quantified by HPLC-DAD and it was shown that caffeoyl quinic derivatives were the most abundant compounds. Chemometric analysis was performed using principal component analysis (PCA) and hierarchical cluster analysis (HCA) based on the qualitative and quantitative LC data. The score plot discriminates different Echinopsis spinosus organs into three distinct clusters, with the stems and flowers allocated in the same cluster, reflecting their resemblance in their secondary metabolites. The antioxidant activities of the methanol extracts were assessed using cupric reducing antioxidant capacity (CUPRAC), ferric reducing antioxidant assay (FRAP), diphenyl picryl hydrazyl radical-scavenging capacity assay (DPPH●), and 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS●+). The root extract exhibited the highest antioxidant activity, evidenced by 3.26 and 1.61 mmol Fe2+/g dried residue for CUPRAC and FRAP, respectively, and great free radical-scavenging activities estimated by 0.53 and 0.82 mmol TEAC/g dried residue for DPPH● and ABTS●+, respectively. The methanol extract of the roots demonstrated a significant level of total phenolics (TP: 125.16 mg GAE/g dried residue) and flavonoids (TFI: 25.40 QE/g dried residue TFII: 140 CE/g dried residue). Molecular docking revealed that tricaffeoyl-altraric acid and dicaffeoyl-altraric acid exhibited the best fit within the active sites of NADPH oxidase (NO) and myeloperoxidase (MP). From ADME/TOPAKT analyses, it can be concluded that tricaffeoyl-altraric acid and dicaffeoyl-altraric acid also revealed reasonable pharmacokinetic and pharmacodynamic characteristics with a significant safety profile.
Keywords: ADME/TOPAKT; Echinops; HPLC-DAD; antioxidant activity; chemometrics; phenolics.
Conflict of interest statement
One of the authors (G.A.C.) is from Natural Products Inc., Evanston, IL 60202, USA, the company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Screening of Antiglaucoma, Antidiabetic, Anti-Alzheimer, and Antioxidant Activities of Astragalus alopecurus Pall-Analysis of Phenolics Profiles by LC-MS/MS.Pharmaceuticals (Basel). 2023 Apr 28;16(5):659. doi: 10.3390/ph16050659. Pharmaceuticals (Basel). 2023. PMID: 37242442 Free PMC article.
-
Chemometric Approach to the Composition of Flavonoid Compounds and Phenolic Acids and Antioxidant Potential of Artemisia Species from Different Habitats.Chem Biodivers. 2022 Dec;19(12):e202200365. doi: 10.1002/cbdv.202200365. Epub 2022 Nov 16. Chem Biodivers. 2022. PMID: 36315629
-
Phenolic compounds analysis of three Euphorbia species by LC-DAD-MSn and their biological properties.J Pharm Biomed Anal. 2020 Sep 10;189:113477. doi: 10.1016/j.jpba.2020.113477. Epub 2020 Jul 15. J Pharm Biomed Anal. 2020. PMID: 32693205
-
Antioxidant activities and phytochemical constituents of Antidesma thwaitesianum Müll. Arg. leaf extracts.J Integr Med. 2017 Jul;15(4):310-319. doi: 10.1016/S2095-4964(17)60334-0. J Integr Med. 2017. PMID: 28659236
-
Total Phenolics, Total Flavonoids, Antioxidant Capacities, and Volatile Compounds Gas Chromatography-Mass Spectrometry Profiling of Moringa oleifera Ripe Seed Polar Fractions.Pharmacogn Mag. 2018 Apr-Jun;14(54):191-194. doi: 10.4103/pm.pm_212_17. Epub 2018 Apr 10. Pharmacogn Mag. 2018. PMID: 29720830 Free PMC article.
Cited by
-
The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties.Molecules. 2022 Apr 27;27(9):2797. doi: 10.3390/molecules27092797. Molecules. 2022. PMID: 35566148 Free PMC article.
-
GC/MS profiling of essential oils from Bontia daphnoides L., chemometric discrimination, isolation of dehydroepingaione and evaluation of antiviral activity.Sci Rep. 2022 Oct 21;12(1):17707. doi: 10.1038/s41598-022-22174-4. Sci Rep. 2022. PMID: 36271233 Free PMC article.
-
Antioxidant and Hepatoprotective Potential of Echinops ritro L. Extracts on Induced Oxidative Stress In Vitro/In Vivo.Int J Mol Sci. 2023 Jun 10;24(12):9999. doi: 10.3390/ijms24129999. Int J Mol Sci. 2023. PMID: 37373147 Free PMC article.
-
Chemical Profiling and Evaluation of Antioxidant Activity of Artichoke (Cynara cardunculus var. scolymus) Leaf By-Products' Extracts Obtained with Green Extraction Techniques.Molecules. 2024 Oct 11;29(20):4816. doi: 10.3390/molecules29204816. Molecules. 2024. PMID: 39459185 Free PMC article.
-
Evaluation of Possible Antioxidant, Anti-Hyperglycaemic, Anti-Alzheimer and Anti-Inflammatory Effects of Teucrium polium Aerial Parts (Lamiaceae).Life (Basel). 2022 Oct 11;12(10):1579. doi: 10.3390/life12101579. Life (Basel). 2022. PMID: 36295014 Free PMC article.
References
-
- Fathy S., Emam M., Agwa S.A., Zahra F.A., Youssef F., Sami R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016;62:80–84. - PubMed
-
- Yoshikawa T., Naito Y. What is oxidative stress? Japan Med. Assoc. J. 2002;45:271–276.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

