Molecular Signature of Astrocytes for Gene Delivery by the Synthetic Adeno-Associated Viral Vector rAAV9P1
- PMID: 35398994
- PMCID: PMC9165502
- DOI: 10.1002/advs.202104979
Molecular Signature of Astrocytes for Gene Delivery by the Synthetic Adeno-Associated Viral Vector rAAV9P1
Abstract
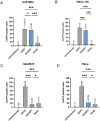
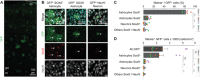
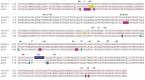
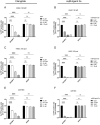
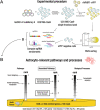
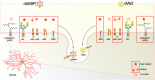
Astrocytes have crucial functions in the central nervous system (CNS) and are major players in many CNS diseases. Research on astrocyte-centered diseases requires efficient and well-characterized gene transfer vectors. Vectors derived from the Adeno-associated virus serotype 9 (AAV9) _target astrocytes in the brains of rodents and nonhuman primates. A recombinant (r) synthetic peptide-displaying AAV9 variant, rAAV9P1, that efficiently and selectively transduces cultured human astrocytes, has been described previously. Here, it is shown that rAAV9P1 retains astrocyte-_targeting properties upon intravenous injection in mice. Detailed analysis of putative receptors on human astrocytes shows that rAAV9P1 utilizes integrin subunits αv, β8, and either β3 or β5 as well as the AAV receptor AAVR. This receptor pattern is distinct from that of vectors derived from wildtype AAV2 or AAV9. Furthermore, a CRISPR/Cas9 genome-wide knockout screening revealed the involvement of several astrocyte-associated intracellular signaling pathways in the transduction of human astrocytes by rAAV9P1. This study delineates the unique receptor and intracellular pathway signatures utilized by rAAV9P1 for _targeting human astrocytes. These results enhance the understanding of the transduction biology of synthetic rAAV vectors for astrocytes and can promote the development of advanced astrocyte-selective gene delivery vehicles for research and clinical applications.
Keywords: AAV; Adeno-associated virus; astrocytes; integrins; receptor profile; vectors.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
D.G. is a co‐founder of the company AaviGen GmbH. D.G. and J.E.A are inventors on a pending patent application (International application number: PCT/EP2019/060790; Publication number: WO/2019/207132) covering AAVMYO and P1 peptide.
Figures
Similar articles
-
Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection.PLoS One. 2017 Dec 15;12(12):e0188830. doi: 10.1371/journal.pone.0188830. eCollection 2017. PLoS One. 2017. PMID: 29244806 Free PMC article.
-
Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain.Neurosci Lett. 2018 Feb 5;665:182-188. doi: 10.1016/j.neulet.2017.11.049. Epub 2017 Nov 24. Neurosci Lett. 2018. PMID: 29175632
-
Intracerebroventricular injection of adeno-associated virus 6 and 9 vectors for cell type-specific transgene expression in the spinal cord.Hum Gene Ther. 2014 Feb;25(2):109-20. doi: 10.1089/hum.2013.021. Epub 2014 Jan 15. Hum Gene Ther. 2014. PMID: 24191919
-
Clinical gene therapy using recombinant adeno-associated virus vectors.Gene Ther. 2008 Jun;15(11):858-63. doi: 10.1038/gt.2008.68. Epub 2008 Apr 17. Gene Ther. 2008. PMID: 18418415 Review.
-
Intracellular trafficking of adeno-associated viral vectors.Gene Ther. 2005 Jun;12(11):873-80. doi: 10.1038/sj.gt.3302527. Gene Ther. 2005. PMID: 15829993 Review.
Cited by
-
An engineered AAV _targeting integrin alpha V beta 6 presents improved myotropism across species.Nat Commun. 2024 Sep 11;15(1):7965. doi: 10.1038/s41467-024-52002-4. Nat Commun. 2024. PMID: 39261465 Free PMC article.
-
Semirational bioengineering of AAV vectors with increased potency and specificity for systemic gene therapy of muscle disorders.Sci Adv. 2022 Sep 23;8(38):eabn4704. doi: 10.1126/sciadv.abn4704. Epub 2022 Sep 21. Sci Adv. 2022. PMID: 36129972 Free PMC article.
-
Dual-_targeting AAV9P1-mediated neuronal reprogramming in a mouse model of traumatic brain injury.Neural Regen Res. 2024 Mar;19(3):629-635. doi: 10.4103/1673-5374.380907. Neural Regen Res. 2024. PMID: 37721294 Free PMC article.
-
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression.Int J Nanomedicine. 2024 Jul 29;19:7691-7708. doi: 10.2147/IJN.S459905. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099791 Free PMC article. Review.
-
Astrocyte-Mediated Neuroinflammation in Neurological Conditions.Biomolecules. 2024 Sep 25;14(10):1204. doi: 10.3390/biom14101204. Biomolecules. 2024. PMID: 39456137 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
