Extracellular Lipids in the Lung and Their Role in Pulmonary Fibrosis
- PMID: 35406772
- PMCID: PMC8997955
- DOI: 10.3390/cells11071209
Extracellular Lipids in the Lung and Their Role in Pulmonary Fibrosis
Abstract
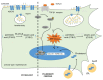
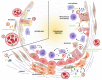
Lipids are major actors and regulators of physiological processes within the lung. Initial research has described their critical role in tissue homeostasis and in orchestrating cellular communication to allow respiration. Over the past decades, a growing body of research has also emphasized how lipids and their metabolism may be altered, contributing to the development and progression of chronic lung diseases such as pulmonary fibrosis. In this review, we first describe the current working model of the mechanisms of lung fibrogenesis before introducing lipids and their cellular metabolism. We then summarize the evidence of altered lipid homeostasis during pulmonary fibrosis, focusing on their extracellular forms. Finally, we highlight how lipid _targeting may open avenues to develop therapeutic options for patients with lung fibrosis.
Keywords: extracellular lipids; idiopathic pulmonary fibrosis; lipid metabolism.
Conflict of interest statement
O.B. and S.L. declare no conflict of interest. G.B. and P.B. received support from Boeringher Ingelheim and Roche for attending medical and research meetings and serving in advisory board.
Figures
Similar articles
-
Lipids - two sides of the same coin in lung fibrosis.Cell Signal. 2019 Aug;60:65-80. doi: 10.1016/j.cellsig.2019.04.007. Epub 2019 Apr 15. Cell Signal. 2019. PMID: 30998969 Review.
-
Roles of lipid metabolism and its regulatory mechanism in idiopathic pulmonary fibrosis: A review.Int J Biochem Cell Biol. 2023 Feb;155:106361. doi: 10.1016/j.biocel.2022.106361. Epub 2022 Dec 31. Int J Biochem Cell Biol. 2023. PMID: 36592687 Review.
-
_targeting Alveolar Repair in Idiopathic Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2021 Oct;65(4):347-365. doi: 10.1165/rcmb.2020-0476TR. Am J Respir Cell Mol Biol. 2021. PMID: 34129811 Free PMC article. Review.
-
Alveolar lipids in pulmonary disease. A review.Lipids Health Dis. 2020 Jun 3;19(1):122. doi: 10.1186/s12944-020-01278-8. Lipids Health Dis. 2020. PMID: 32493486 Free PMC article. Review.
-
The Role of Extracellular Vesicles in Idiopathic Pulmonary Fibrosis Progression: An Approach on Their Therapeutics Potential.Cells. 2022 Feb 11;11(4):630. doi: 10.3390/cells11040630. Cells. 2022. PMID: 35203281 Free PMC article. Review.
Cited by
-
Multiomics analysis reveals the mechanical stress-dependent changes in trabecular meshwork cytoskeletal-extracellular matrix interactions.Front Cell Dev Biol. 2022 Sep 13;10:874828. doi: 10.3389/fcell.2022.874828. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36176278 Free PMC article.
-
Effects of Anti-Fibrotic Drugs on Transcriptome of Peripheral Blood Mononuclear Cells in Idiopathic Pulmonary Fibrosis.Int J Mol Sci. 2024 Mar 28;25(7):3750. doi: 10.3390/ijms25073750. Int J Mol Sci. 2024. PMID: 38612561 Free PMC article.
-
CT-based body composition analysis and pulmonary fat attenuation volume as biomarkers to predict overall survival in patients with non-specific interstitial pneumonia.Eur Radiol Exp. 2024 Oct 14;8(1):114. doi: 10.1186/s41747-024-00519-0. Eur Radiol Exp. 2024. PMID: 39400764 Free PMC article.
-
Downregulation of HMGCS2 mediated AECIIs lipid metabolic alteration promotes pulmonary fibrosis by activating fibroblasts.Respir Res. 2024 Apr 24;25(1):176. doi: 10.1186/s12931-024-02816-z. Respir Res. 2024. PMID: 38658970 Free PMC article.
-
Beyond the Acute Phase: Long-Term Impact of COVID-19 on Functional Capacity and Prothrombotic Risk-A Pilot Study.Medicina (Kaunas). 2023 Dec 27;60(1):51. doi: 10.3390/medicina60010051. Medicina (Kaunas). 2023. PMID: 38256314 Free PMC article.
References
-
- Richeldi L., Cottin V., du Bois R.M., Selman M., Kimura T., Bailes Z., Schlenker-Herceg R., Stowasser S., Brown K.K. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS(®) trials. Respir. Med. 2016;113:74–79. doi: 10.1016/j.rmed.2016.02.001. - DOI - PubMed
-
- King T.E., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

