In Vitro Cancer Models: A Closer Look at Limitations on Translation
- PMID: 35447726
- PMCID: PMC9029854
- DOI: 10.3390/bioengineering9040166
In Vitro Cancer Models: A Closer Look at Limitations on Translation
Abstract
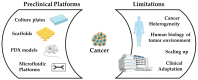
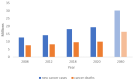
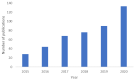
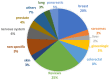
In vitro cancer models are envisioned as high-throughput screening platforms for potential new therapeutic discovery and/or validation. They also serve as tools to achieve personalized treatment strategies or real-time monitoring of disease propagation, providing effective treatments to patients. To battle the fatality of metastatic cancers, the development and commercialization of predictive and robust preclinical in vitro cancer models are of urgent need. In the past decades, the translation of cancer research from 2D to 3D platforms and the development of diverse in vitro cancer models have been well elaborated in an enormous number of reviews. However, the meagre clinical success rate of cancer therapeutics urges the critical introspection of currently available preclinical platforms, including patents, to hasten the development of precision medicine and commercialization of in vitro cancer models. Hence, the present article critically reflects the difficulty of translating cancer therapeutics from discovery to adoption and commercialization in the light of in vitro cancer models as predictive tools. The state of the art of in vitro cancer models is discussed first, followed by identifying the limitations of bench-to-bedside transition. This review tries to establish compatibility between the current findings and obstacles and indicates future directions to accelerate the market penetration, considering the niche market.
Keywords: 3D cancer models; cancer; commercialization; gap analysis; point-of-care modelling tool.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Microengineered 3D Tumor Models for Anti-Cancer Drug Discovery in Female-Related Cancers.Ann Biomed Eng. 2021 Aug;49(8):1943-1972. doi: 10.1007/s10439-020-02704-9. Epub 2021 Jan 5. Ann Biomed Eng. 2021. PMID: 33403451 Review.
-
Microengineered Organ-on-a-chip Platforms towards Personalized Medicine.Curr Pharm Des. 2018;24(45):5354-5366. doi: 10.2174/1381612825666190222143542. Curr Pharm Des. 2018. PMID: 30799783 Review.
-
Skin-on-a-chip technologies towards clinical translation and commercialization.Biofabrication. 2024 Jul 16;16(4). doi: 10.1088/1758-5090/ad5f55. Biofabrication. 2024. PMID: 38964314 Review.
-
Could 3D models of cancer enhance drug screening?Biomaterials. 2020 Feb;232:119744. doi: 10.1016/j.biomaterials.2019.119744. Epub 2019 Dec 26. Biomaterials. 2020. PMID: 31918229 Review.
-
One mouse, one patient paradigm: New avatars of personalized cancer therapy.Cancer Lett. 2014 Mar 1;344(1):1-12. doi: 10.1016/j.canlet.2013.10.010. Epub 2013 Oct 22. Cancer Lett. 2014. PMID: 24157811 Free PMC article. Review.
Cited by
-
Prostate Cancer Organoids for Tumor Modeling and Drug Screening.Methods Mol Biol. 2024;2777:135-144. doi: 10.1007/978-1-0716-3730-2_10. Methods Mol Biol. 2024. PMID: 38478341
-
The Power of Gene Technologies: 1001 Ways to Create a Cell Model.Cells. 2022 Oct 14;11(20):3235. doi: 10.3390/cells11203235. Cells. 2022. PMID: 36291103 Free PMC article. Review.
-
Differential Effects of Iron Chelates vs. Iron Salts on Induction of Pro-Oncogenic Amphiregulin and Pro-Inflammatory COX-2 in Human Intestinal Adenocarcinoma Cell Lines.Int J Mol Sci. 2023 Mar 14;24(6):5507. doi: 10.3390/ijms24065507. Int J Mol Sci. 2023. PMID: 36982582 Free PMC article.
-
Green Routes for Bio-Fabrication in Biomedical and Pharmaceutical Applications.Pharmaceutics. 2023 Jun 15;15(6):1744. doi: 10.3390/pharmaceutics15061744. Pharmaceutics. 2023. PMID: 37376192 Free PMC article. Review.
-
Fisetin-loaded nanoemulsion ameliorates lung cancer pathogenesis via downregulating cathepsin-B, galectin-3 and enolase in an in vitro setting.EXCLI J. 2024 Oct 14;23:1238-1244. doi: 10.17179/excli2024-7583. eCollection 2024. EXCLI J. 2024. PMID: 39574963 Free PMC article. No abstract available.
References
-
- Cancer Tomorrow. [(accessed on 11 May 2021)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?types=0&single_unit=500000.
Publication types
Grants and funding
- FROnTHERA-NORTE-01-0145-FEDER-000023/CCDR-N - Northern Regional Development and Coordination Commission; NORTE2020; FEDER - Fundo Europeu de Desenvolvimento Regional
- nº 668983 - FoReCaST/European Union Framework Programme for Research and Innovation Horizon 2020
- PD/BD/143050/2018/Fundação para a Ciência e Tecnologia
- PTDC/BTM-ORG/28168/2017/Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources
Miscellaneous

