Production, purification, and characterization of recombinant rabies virus glycoprotein expressed in PichiaPink™ yeast
- PMID: 35646619
- PMCID: PMC9130087
- DOI: 10.1016/j.btre.2022.e00736
Production, purification, and characterization of recombinant rabies virus glycoprotein expressed in PichiaPink™ yeast
Abstract
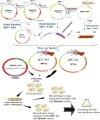
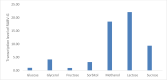
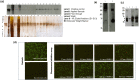
The commonly used host for industrial production of recombinant proteins Pichia pastoris, has been used in this work to produce the rabies virus glycoprotein (RABV-G). To allow a constitutive expression and the secretion of the expressed recombinant RABV-G, the PichiaPink™ commercialized expression vectors were modified to contain the constitutive GAP promoter and the α secretion signal sequences. Recombinant PichiaPink™ strains co-expressing the RABV-G and the protein chaperone PDI, have been then generated and screened for the best producer clone. The influence of seven carbon sources on the expression of the RABV-G, has been studied under different culture conditions in shake flask culture. An incubation temperature of 30°C under an agitation rate of 250 rpm in a filling volume of 10:1 flask/culture volume ratio were the optimal conditions for the RABV-G production in shake flask for all screened carbon sources. A bioreactor Fed batch culture has been then carried using glycerol and glucose as they were good carbon sources for cell growth and RABV-G production in shake flask scale. Cells were grown on glycerol during the batch phase then fed with glycerol or glucose defined solutions, a final RABV-G concentration of 2.7 µg/l was obtained with a specific product yield (YP/X) of 0.032 and 0.06 µg/g(DCW) respectively. The use of semi-defined feeding solution enhanced the production and the YP/X to 12.9 µg/l and 0.135 µg/g(DCW) respectively. However, the high cell density favored by these carbon sources resulted in oxygen limitation which influenced the glycosylation pattern of the secreted RABV-G. Alternatively, the use of sucrose as substrate for RABV-G production in large scale culture, resulted in less biomass production and a YP/X of 0.310 µg/g(DCW) was obtained. A cation exchange chromatography was then used for RABV-G purification as one step method. The purified protein was correctly folded and glycosylated and able to adopt trimeric conformation. The knowledges gained through this work offer a valuable insight into the bioprocess design of RABV-G production in Pichia pastoris to obtain a correctly folded protein which can be used during an immunization proposal for subunit Rabies vaccine development.
Keywords: Carbon source; Fed-batch fermentation; Pichia pastoris constitutive expression; Rabies virus glycoprotein.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Bioprocess and downstream optimization of recombinant bovine chymosin B in Pichia (Komagataella) pastoris under methanol-inducible AOXI promoter.Protein Expr Purif. 2014 Dec;104:85-91. doi: 10.1016/j.pep.2014.09.014. Epub 2014 Sep 30. Protein Expr Purif. 2014. PMID: 25278015
-
Enhancement of thermoalkaliphilic xylanase production by Pichia pastoris through novel fed-batch strategy in high cell-density fermentation.BMC Biotechnol. 2017 Jun 21;17(1):55. doi: 10.1186/s12896-017-0361-6. BMC Biotechnol. 2017. PMID: 28633643 Free PMC article.
-
Functional recombinant protein is present in the pre-induction phases of Pichia pastoris cultures when grown in bioreactors, but not shake-flasks.Microb Cell Fact. 2014 Sep 4;13(1):127. doi: 10.1186/s12934-014-0127-y. Microb Cell Fact. 2014. PMID: 25186468 Free PMC article.
-
Constitutive expression of Yarrowia lipolytica lipase LIP2 in Pichia pastoris using GAP as promoter.Appl Biochem Biotechnol. 2012 Mar;166(5):1355-67. doi: 10.1007/s12010-011-9524-4. Epub 2012 Jan 14. Appl Biochem Biotechnol. 2012. PMID: 22246727
-
Secretory expression of human protein in the Yeast Pichia pastoris by controlled fermentor culture.Recent Pat Biotechnol. 2010 Jun;4(2):153-66. doi: 10.2174/187220810791110679. Recent Pat Biotechnol. 2010. PMID: 20180764 Review.
Cited by
-
High yield expression and purification of Aspergillus flavus uricase cloned in Pichia pink™ expression system.3 Biotech. 2023 Sep;13(9):306. doi: 10.1007/s13205-023-03710-z. Epub 2023 Aug 19. 3 Biotech. 2023. PMID: 37605761 Free PMC article.
-
An mRNA vaccine against rabies provides strong and durable protection in mice.Front Immunol. 2023 Oct 26;14:1288879. doi: 10.3389/fimmu.2023.1288879. eCollection 2023. Front Immunol. 2023. PMID: 37954577 Free PMC article.
References
-
- Puetz J., Wurm F.M. Recombinant proteins for industrial versus pharmaceutical purposes: a review of process and pricing. Processes. 2019;7(8):476.
-
- Damasceno L.M., Huang C.J., Batt C.A. Protein secretion in Pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 2012;93(1):31–39. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
