Identification of ADS024, a newly characterized strain of Bacillus velezensis with direct Clostridiodes difficile killing and toxin degradation bio-activities
- PMID: 35662257
- PMCID: PMC9166764
- DOI: 10.1038/s41598-022-13248-4
Identification of ADS024, a newly characterized strain of Bacillus velezensis with direct Clostridiodes difficile killing and toxin degradation bio-activities
Abstract
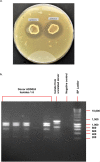
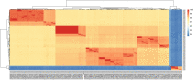
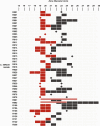
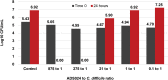
Clostridioides difficile infection (CDI) remains a significant health threat worldwide. C. difficile is an opportunistic, toxigenic pathogen that takes advantage of a disrupted gut microbiome to grow and produce signs and symptoms ranging from diarrhea to pseudomembranous colitis. Antibiotics used to treat C. difficile infection are usually broad spectrum and can further disrupt the commensal gut microbiota, leaving patients susceptible to recurrent C. difficile infection. There is a growing need for therapeutic options that can continue to inhibit the outgrowth of C. difficile after antibiotic treatment is completed. Treatments that degrade C. difficile toxins while having minimal collateral impact on gut bacteria are also needed to prevent recurrence. Therapeutic bacteria capable of producing a range of antimicrobial compounds, proteases, and other bioactive metabolites represent a potentially powerful tool for preventing CDI recurrence following resolution of symptoms. Here, we describe the identification and initial characterization of ADS024 (formerly ART24), a novel therapeutic bacterium that can kill C. difficile in vitro with limited impact on other commensal bacteria. In addition to directly killing C. difficile, ADS024 also produces proteases capable of degrading C. difficile toxins, the drivers of symptoms associated with most cases of CDI. ADS024 is in clinical development for the prevention of CDI recurrence as a single-strain live biotherapeutic product, and this initial data set supports further studies aimed at evaluating ADS024 in future human clinical trials.
© 2022. The Author(s).
Conflict of interest statement
The authors of this manuscript have several competing interests. PR, CH, and MCR received consulting fees from Adiso Therapeutics, Inc. LC and RF are employees of Adiso Therapeutics, Inc. MMOD, BH, JH, SS, CJW are supported by an Adiso Therapeutics, Inc. research grant to UCC/APC. All authors declare that they have no non-financial interests.
Figures


Similar articles
-
Novel, non-colonizing, single-strain live biotherapeutic product ADS024 protects against Clostridioides difficile infection challenge in vivo.World J Gastrointest Pathophysiol. 2023 Aug 24;14(4):71-85. doi: 10.4291/wjgp.v14.i4.71. World J Gastrointest Pathophysiol. 2023. PMID: 37727283 Free PMC article.
-
Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection.Drugs. 2022 Oct;82(15):1527-1538. doi: 10.1007/s40265-022-01797-x. Epub 2022 Oct 26. Drugs. 2022. PMID: 36287379 Free PMC article. Clinical Trial.
-
SER-109 (VOWST™): A Review in the Prevention of Recurrent Clostridioides difficile Infection.Drugs. 2024 Mar;84(3):329-336. doi: 10.1007/s40265-024-02006-7. Epub 2024 Mar 5. Drugs. 2024. PMID: 38441806 Review.
-
ADS024, a Bacillus velezensis strain, protects human colonic epithelial cells against C. difficile toxin-mediated apoptosis.Front Microbiol. 2023 Jan 10;13:1072534. doi: 10.3389/fmicb.2022.1072534. eCollection 2022. Front Microbiol. 2023. PMID: 36704560 Free PMC article.
-
Ridinilazole: a novel, narrow-spectrum antimicrobial agent _targeting Clostridium (Clostridioides) difficile.Lett Appl Microbiol. 2022 Sep;75(3):526-536. doi: 10.1111/lam.13664. Epub 2022 Feb 11. Lett Appl Microbiol. 2022. PMID: 35119124 Free PMC article. Review.
Cited by
-
Clostridioides difficile infection: microbe-microbe interactions and live biotherapeutics.Front Microbiol. 2023 May 9;14:1182612. doi: 10.3389/fmicb.2023.1182612. eCollection 2023. Front Microbiol. 2023. PMID: 37228365 Free PMC article. Review.
-
Bacillus velezensis DSM 33864 reduces Clostridioides difficile colonization without disturbing commensal gut microbiota composition.Sci Rep. 2023 Sep 11;13(1):14941. doi: 10.1038/s41598-023-42128-8. Sci Rep. 2023. PMID: 37696924 Free PMC article.
-
An Intestinal Bacillus velezensis Isolate Displays Broad-Spectrum Antibacterial Activity and Prevents Infection of Both Gram-Positive and Gram-Negative Pathogens In Vivo.J Bacteriol. 2023 Jun 27;205(6):e0013323. doi: 10.1128/jb.00133-23. Epub 2023 May 17. J Bacteriol. 2023. PMID: 37195186 Free PMC article.
-
The Urgent Threat of Clostridioides difficile Infection: A Glimpse of the Drugs of the Future, with Related Patents and Prospects.Biomedicines. 2023 Feb 1;11(2):426. doi: 10.3390/biomedicines11020426. Biomedicines. 2023. PMID: 36830964 Free PMC article. Review.
-
Genetic and Phenotypic Characterization of Bacillus velezensis Strain BV379 for Human Probiotic Applications.Microorganisms. 2024 Feb 21;12(3):436. doi: 10.3390/microorganisms12030436. Microorganisms. 2024. PMID: 38543487 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

