The Potential of Epigallocatechin Gallate (EGCG) in _targeting Autophagy for Cancer Treatment: A Narrative Review
- PMID: 35682754
- PMCID: PMC9181147
- DOI: 10.3390/ijms23116075
The Potential of Epigallocatechin Gallate (EGCG) in _targeting Autophagy for Cancer Treatment: A Narrative Review
Abstract
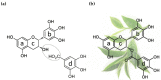
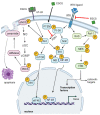
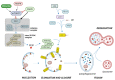
Autophagy is an evolutionarily conserved process for the degradation of redundant or damaged cellular material by means of a lysosome-dependent mechanism, contributing to cell homeostasis and survival. Autophagy plays a multifaceted and context-dependent role in cancer initiation, maintenance, and progression; it has a tumor suppressive role in the absence of disease and is upregulated in cancer cells to meet their elevated metabolic demands. Autophagy represents a promising but challenging _target in cancer treatment. Green tea is a widely used beverage with healthy effects on several diseases, including cancer. The bioactive compounds of green tea are mainly catechins, and epigallocatechin-gallate (EGCG) is the most abundant and biologically active among them. In this review, evidence of autophagy modulation and anti-cancer effects induced by EGCG treatment in experimental cancer models is presented. Reviewed articles reveal that EGCG promotes cytotoxic autophagy often through the inactivation of PI3K/Akt/mTOR pathway, resulting in apoptosis induction. EGCG pro-oxidant activity has been postulated to be responsible for its anti-cancer effects. In combination therapy with a chemotherapy drug, EGCG inhibits cell growth and the drug-induced pro-survival autophagy. The selected studies rightly claim EGCG as a valuable agent in cancer chemoprevention.
Keywords: autophagy; autophagy activator; autophagy modulator; cancer therapy; epigallocatechin gallate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Epigallocatechin-3-gallate induces autophagy-related apoptosis associated with LC3B II and Beclin expression of bladder cancer cells.J Food Biochem. 2021 Jun;45(6):e13758. doi: 10.1111/jfbc.13758. Epub 2021 May 16. J Food Biochem. 2021. PMID: 33997996
-
Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress.Oxid Med Cell Longev. 2018 Jan 31;2018:6721530. doi: 10.1155/2018/6721530. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29636854 Free PMC article.
-
Molecular _targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer.Nutrients. 2018 Dec 6;10(12):1936. doi: 10.3390/nu10121936. Nutrients. 2018. PMID: 30563268 Free PMC article. Review.
-
Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications.Molecules. 2023 Jul 6;28(13):5246. doi: 10.3390/molecules28135246. Molecules. 2023. PMID: 37446908 Free PMC article. Review.
-
New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate.Redox Biol. 2014 Jan 10;2:187-95. doi: 10.1016/j.redox.2013.12.022. eCollection 2014. Redox Biol. 2014. PMID: 24494192 Free PMC article. Review.
Cited by
-
Polyphenol Mediated Suppression of Hepatocellular Carcinoma (HepG2) Cell Proliferation by Clerodendrum infortunatum L. Root.Asian Pac J Cancer Prev. 2024 Jan 1;25(1):351-363. doi: 10.31557/APJCP.2024.25.1.351. Asian Pac J Cancer Prev. 2024. PMID: 38285803 Free PMC article.
-
A recent update on the connection between dietary phytochemicals and skin cancer: emerging understanding of the molecular mechanism.Ann Med Surg (Lond). 2024 Aug 7;86(10):5877-5913. doi: 10.1097/MS9.0000000000002392. eCollection 2024 Oct. Ann Med Surg (Lond). 2024. PMID: 39359831 Free PMC article. Review.
-
Unraveling cancer progression pathways and phytochemical therapeutic strategies for its management.Front Pharmacol. 2024 Aug 23;15:1414790. doi: 10.3389/fphar.2024.1414790. eCollection 2024. Front Pharmacol. 2024. PMID: 39246660 Free PMC article. Review.
-
Modulating autophagy and mitophagy as a promising therapeutic approach in neurodegenerative disorders.Life Sci. 2022 Dec 15;311(Pt A):121153. doi: 10.1016/j.lfs.2022.121153. Epub 2022 Nov 4. Life Sci. 2022. PMID: 36343743 Free PMC article. Review.
-
Polyphenols as Potent Epigenetics Agents for Cancer.Int J Mol Sci. 2022 Oct 3;23(19):11712. doi: 10.3390/ijms231911712. Int J Mol Sci. 2022. PMID: 36233012 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

