Comparison of BNT162b2-, mRNA-1273- and Ad26.COV2.S-Elicited IgG and Neutralizing Titers against SARS-CoV-2 and Its Variants
- PMID: 35746466
- PMCID: PMC9228110
- DOI: 10.3390/vaccines10060858
Comparison of BNT162b2-, mRNA-1273- and Ad26.COV2.S-Elicited IgG and Neutralizing Titers against SARS-CoV-2 and Its Variants
Abstract
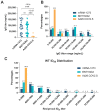
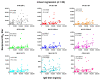
Because the vaccine-elicited antibody and neutralizing activity against spike protein of SARS-CoV-2 are associated with protection from COVID-19, it is important to determine the levels of specific IgG and neutralization titers against SARS-CoV-2 elicited by the vaccines. While three widely used vaccine brands (Pfizer-BNT162b2, Moderna-mRNA-1273 and Johnson-Ad26.COV2.S) are effective in preventing SARS-CoV-2 infection and alleviating COVID-19 illness, they have different efficacy against COVID-19. It is unclear whether the differences are due to varying ability of the vaccines to elicit a specific IgG antibody response and neutralization activity against spike protein of the virus. In this study, we compared the plasma IgG and neutralization titers against spike proteins of wild-type SARS-CoV-2 and eight variants in healthy subjects who received the mRNA-1273, BNT162b2 or Ad26.COV2.S vaccine. We demonstrated that subjects vaccinated with Ad26.COV2.S vaccine had significantly lower levels of IgG and neutralizing titers as compared to those who received the mRNA vaccines. While the linear regression analysis showed a positive correlation between IgG levels and neutralizing activities against SARS-CoV-2 WT and the variants, there was an overall reduction in neutralizing titers against the variants in subjects across the three groups. These findings suggest that people who received one dose of Ad26.COV2.S vaccine have a more limited IgG response and lower neutralization activity against SARS-CoV-2 WT and its variants than recipients of the mRNA vaccines. Thus, monitoring the plasma or serum levels of anti-SARS-CoV-2 spike IgG titer and neutralization activity is necessary for the selection of suitable vaccines, vaccine dosage and regimens.
Keywords: Ad26.COV2.S; BNT162b2; SARS-CoV-2; antibody; mRNA-1273; neutralization; vaccine; variants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection.Front Immunol. 2023 Mar 7;14:1131229. doi: 10.3389/fimmu.2023.1131229. eCollection 2023. Front Immunol. 2023. PMID: 36960070 Free PMC article.
-
Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines.Front Med (Lausanne). 2022 Oct 3;9:994160. doi: 10.3389/fmed.2022.994160. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36262278 Free PMC article.
-
Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers.Int J Infect Dis. 2022 Oct;123:183-191. doi: 10.1016/j.ijid.2022.08.022. Epub 2022 Aug 28. Int J Infect Dis. 2022. PMID: 36044963 Free PMC article.
-
SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines.Vaccines (Basel). 2021 Mar 5;9(3):227. doi: 10.3390/vaccines9030227. Vaccines (Basel). 2021. PMID: 33807818 Free PMC article. Review.
-
Immunogenicity and clinical features relating to BNT162b2 messenger RNA COVID-19 vaccine, Ad26.COV2.S and ChAdOx1 adenoviral vector COVID-19 vaccines: a systematic review of non-interventional studies.Futur J Pharm Sci. 2022;8(1):20. doi: 10.1186/s43094-022-00409-5. Epub 2022 Mar 26. Futur J Pharm Sci. 2022. PMID: 35368622 Free PMC article. Review.
Cited by
-
Protein expression/secretion boost by a novel unique 21-mer cis-regulatory motif (Exin21) via mRNA stabilization.Mol Ther. 2023 Apr 5;31(4):1136-1158. doi: 10.1016/j.ymthe.2023.02.012. Epub 2023 Feb 14. Mol Ther. 2023. PMID: 36793212 Free PMC article.
-
Pre-existing cell populations with cytotoxic activity against SARS-CoV-2 in people with HIV and normal CD4/CD8 ratio previously unexposed to the virus.Front Immunol. 2024 May 15;15:1362621. doi: 10.3389/fimmu.2024.1362621. eCollection 2024. Front Immunol. 2024. PMID: 38812512 Free PMC article.
-
SARS-CoV-2 Vaccination: What Can We Expect Now?Vaccines (Basel). 2022 Jul 8;10(7):1093. doi: 10.3390/vaccines10071093. Vaccines (Basel). 2022. PMID: 35891257 Free PMC article.
References
-
- COVID-19 Vaccinations in the United States. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

