Refining the migration and engraftment of short-term and long-term HSCs by enhancing homing-specific adhesion mechanisms
- PMID: 35764498
- PMCID: PMC9636332
- DOI: 10.1182/bloodadvances.2022007465
Refining the migration and engraftment of short-term and long-term HSCs by enhancing homing-specific adhesion mechanisms
Abstract
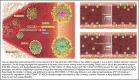
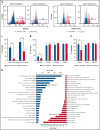
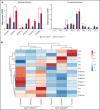
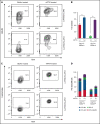
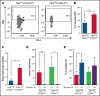
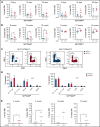
In contrast to the short-term (ST) CD34+ stem cells, studies have suggested that long-term (LT) hematopoietic stem cells (HSCs) found in the CD34- stem cell pool have trouble migrating and engrafting when introduced through IV. To understand why these deficiencies exist, we set out to fully elucidate the adhesion mechanisms used by ST and LT-HSCs to migrate to the bone marrow(BM). Specifically focusing on murine ST-HSCs (Flk2-CD34+) and LT-HSCs (Flk2-CD34-), we observed a distinctive expression pattern of BM homing effectors necessary for the first step, namely sialyl Lewis-X (sLex) (ligand for E-selectin), and the second step, namely CXCR4 chemokine receptor (receptor for SDF-1). sLex expression was higher on Flk2-CD34+ ST-HSCs (>60%) compared with Flk2-CD34- LT-HSCs (<10%), which correlated to binding to E-selectin. Higher concentrations of CXCR4 were observed on Flk2-CD34+ ST-HSCs compared with Flk2-CD34- LT-HSCs. Interestingly, the expression of CD26, a peptidase known to deactivate chemokines (ie, SDF-1), was higher on Flk2-CD34- LT-HSCs. Given that both E-selectin-binding and CXCR4-mediated migration are compromised in Flk2-CD34- LT-HSCs, we aimed to enhance their ability to migrate using recombinant human fucosyltransferase 6 (rhFTVI) and the CD26 inhibitor, Dip A (diprotin A). To this end, we observed that although LT-HSCs expressed low concentrations of sLex, they were able to engraft when transplanted into recipient mice. Moreover, although both CD26 inhibition and fucosylation enhanced migration of both HSC populations in vitro, only pretreatment of LT-HSCs with Dip A enhanced engraftment in vivo after transplantation into recipient mice. Remarkably, fucosylation of Flk2-CD34+ ST-HSCs consistently led to their ability to transplant secondary recipients. These data suggest that using fucosylation and Dip A to overcome the molecular disparity in adhesion mechanisms among ST-HSCs and LT-HSCs differentially influences their abilities to migrate and engraft in vivo and promotes the ability of ST-HSCs to engraft secondary recipient mice, the gold standard for testing functionality of LT-HSCs.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


Similar articles
-
Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice.Stem Cells Dev. 2007 Jun;16(3):347-54. doi: 10.1089/scd.2007.9995. Stem Cells Dev. 2007. PMID: 17610364
-
AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis.Ann N Y Acad Sci. 2007 Jun;1106:1-19. doi: 10.1196/annals.1392.013. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360804
-
CD26 inhibition on CD34+ or lineage- human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice.Stem Cells Dev. 2007 Jun;16(3):355-60. doi: 10.1089/scd.2007.9996. Stem Cells Dev. 2007. PMID: 17610365
-
Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions.Ann N Y Acad Sci. 2001 Jun;938:83-95. doi: 10.1111/j.1749-6632.2001.tb03577.x. Ann N Y Acad Sci. 2001. PMID: 11458529 Review.
-
A sticky wicket: Defining molecular functions for CD34 in hematopoietic cells.Exp Hematol. 2020 Jun;86:1-14. doi: 10.1016/j.exphem.2020.05.004. Epub 2020 May 16. Exp Hematol. 2020. PMID: 32422232 Review.
Cited by
-
EPO modified MSCs protects SH-SY5Y cells against ischemia/hypoxia-induced apoptosis via REST-dependent epigenetic remodeling.Sci Rep. 2024 Oct 6;14(1):23252. doi: 10.1038/s41598-024-74261-3. Sci Rep. 2024. PMID: 39370424 Free PMC article.
-
The Bone Marrow Immune Microenvironment in CML: Treatment Responses, Treatment-Free Remission, and Therapeutic Vulnerabilities.Curr Hematol Malig Rep. 2023 Apr;18(2):19-32. doi: 10.1007/s11899-023-00688-6. Epub 2023 Feb 13. Curr Hematol Malig Rep. 2023. PMID: 36780103 Free PMC article. Review.
-
Synergistic effect and molecular mechanism of nicotinamide and UM171 in ex vivo expansion of long-term hematopoietic stem cells.Regen Ther. 2024 Mar 27;27:191-199. doi: 10.1016/j.reth.2024.03.011. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38840730 Free PMC article.
-
Nanoscopic Characterization of Cell Migration under Flow Using Optical and Electron Microscopy.Anal Chem. 2023 Jan 10;95(3):1958-66. doi: 10.1021/acs.analchem.2c04222. Online ahead of print. Anal Chem. 2023. PMID: 36627105 Free PMC article.
-
CD34+ HSPCs-derived exosomes contain dynamic cargo and promote their migration through functional binding with the homing receptor E-selectin.Front Cell Dev Biol. 2023 Apr 25;11:1149912. doi: 10.3389/fcell.2023.1149912. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37181754 Free PMC article.
References
-
- Donnelly DS, Zelterman D, Sharkis S, Krause DS. Functional activity of murine CD34+ and CD34- hematopoietic stem cell populations. Exp Hematol. 1999;27(5):788–796. - PubMed
-
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–245. - PubMed
-
- Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3(12):1337–1345. - PubMed
-
- Adolfsson J, Borge OJ, Bryder D, et al. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15(4):659–669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

