Intravenous IgM-enriched immunoglobulins in critical COVID-19: a multicentre propensity-weighted cohort study
- PMID: 35799196
- PMCID: PMC9260992
- DOI: 10.1186/s13054-022-04059-0
Intravenous IgM-enriched immunoglobulins in critical COVID-19: a multicentre propensity-weighted cohort study
Abstract
Background: A profound inflammation-mediated lung injury with long-term acute respiratory distress and high mortality is one of the major complications of critical COVID-19. Immunoglobulin M (IgM)-enriched immunoglobulins seem especially capable of mitigating the inflicted inflammatory harm. However, the efficacy of intravenous IgM-enriched preparations in critically ill patients with COVID-19 is largely unclear.
Methods: In this retrospective multicentric cohort study, 316 patients with laboratory-confirmed critical COVID-19 were treated in ten German and Austrian ICUs between May 2020 and April 2021. The primary outcome was 30-day mortality. Analysis was performed by Cox regression models. Covariate adjustment was performed by propensity score weighting using machine learning-based SuperLearner to overcome the selection bias due to missing randomization. In addition, a subgroup analysis focusing on different treatment regimens and patient characteristics was performed.
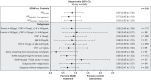
Results: Of the 316 ICU patients, 146 received IgM-enriched immunoglobulins and 170 cases did not, which served as controls. There was no survival difference between the two groups in terms of mortality at 30 days in the overall cohort (HRadj: 0.83; 95% CI: 0.55 to 1.25; p = 0.374). An improved 30-day survival in patients without mechanical ventilation at the time of the immunoglobulin treatment did not reach statistical significance (HRadj: 0.23; 95% CI: 0.05 to 1.08; p = 0.063). Also, no statistically significant difference was observed in the subgroup when a daily dose of ≥ 15 g and a duration of ≥ 3 days of IgM-enriched immunoglobulins were applied (HRadj: 0.65; 95% CI: 0.41 to 1.03; p = 0.068).
Conclusions: Although we cannot prove a statistically reliable effect of intravenous IgM-enriched immunoglobulins, the confidence intervals may suggest a clinically relevant effect in certain subgroups. Here, an early administration (i.e. in critically ill but not yet mechanically ventilated COVID-19 patients) and a dose of ≥ 15 g for at least 3 days may confer beneficial effects without concerning safety issues. However, these findings need to be validated in upcoming randomized clinical trials. Trial registration DRKS00025794 , German Clinical Trials Register, https://www.drks.de . Registered 6 July 2021.
Keywords: COVID-19; Coronavirus disease; Immunoglobulin M; Immunoglobulins; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
TR received a speaker’s honoraria from Biotest AG and grants from Deutsche Forschungsgemeinschaft (DFG) outside of the submitted work. FK has no conflict of interest to declare. HH has no conflict of interest to declare. UA has no conflict of interest to declare. TB has no conflict of interest to declare. HN has no conflict of interest to declare. DJ has no conflict of interest to declare. MD has no conflict of interest to declare. HM received a speaker’s honorarium from CSL Behring (Germany) outside the submitted work. FZ has no conflict of interest to declare. JB has no conflict of interest to declare. CL has no conflict of interest to declare. TK has no conflict of interest to declare. KZ has received honoraria for participation in advisory board meetings for Haemonetics and Vifor and received speaker fees from CSL Behring and GE Healthcare. He is the Principal Investigator of the EU-Horizon 2020 project ENVISION (Intelligent plug-and-play digital tool for real-time surveillance of COVID-19 patients and smart decision-making in Intensive Care Units) and Horizon Europe 2021 project COVend (Biomarker and AI-supported FX06 therapy to prevent progression from mild and moderate to severe stages of COVID-19). MAW received speakers or advisory board honoraria from Biotest, SOBI, Pfizer, Gilead, MSD, Shionogi, Eumedica, BBraun. PR has no conflict of interest to declare. RU received speaker’s honoraria from Biotest AG outside of the submitted work and grants from Apeptico, Bayer and CCORE. He is a founding partner of CCORE, a medical device company. PM received speaker’s honoraria from Biotest AG outside of the submitted work. AN received speaker’s honoraria and travel reimbursement from Biotest AG, and speaker’s honoraria from ThermoFisher Scientific, CyoSorbents and Dräger Medical outside of the submitted work. DKM has received speaker’s honoraria from Biotest AG. NT has no conflict of interest to declare. MA received a speaker’s honoraria from Biotest AG and grants from the State of North Rhine Westphalia outside of the submitted work.
Figures
Similar articles
-
The outcome of using intravenous immunoglobulin (IVIG) in critically ill COVID-19 patients': a retrospective, multi-centric cohort study.Eur J Med Res. 2022 Feb 3;27(1):18. doi: 10.1186/s40001-022-00637-8. Eur J Med Res. 2022. PMID: 35115056 Free PMC article.
-
Effect of early treatment with polyvalent immunoglobulin on acute respiratory distress syndrome associated with SARS-CoV-2 infections (ICAR trial): study protocol for a randomized controlled trial.Trials. 2021 Feb 28;22(1):170. doi: 10.1186/s13063-021-05118-7. Trials. 2021. PMID: 33648563 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Intravenous immunoglobulin as an important adjunct in the prevention and therapy of coronavirus 2019 disease.Scand J Immunol. 2021 Nov;94(5):e13101. doi: 10.1111/sji.13101. Epub 2021 Sep 16. Scand J Immunol. 2021. PMID: 34940980 Free PMC article. Review.
-
Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation.Cochrane Database Syst Rev. 2014 Jul 9;2014(7):CD009176. doi: 10.1002/14651858.CD009176.pub2. Cochrane Database Syst Rev. 2014. PMID: 25005604 Free PMC article. Review.
Cited by
-
Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections-Interactions with Neutrophils, Complement, and Coagulation Pathways.Biomedicines. 2023 Nov 10;11(11):3022. doi: 10.3390/biomedicines11113022. Biomedicines. 2023. PMID: 38002022 Free PMC article. Review.
-
Case Series: Efficacy of Polyclonal Intravenous Immunoglobulin for Refractory Clostridioides difficile Infection.Antibodies (Basel). 2024 Apr 1;13(2):26. doi: 10.3390/antib13020026. Antibodies (Basel). 2024. PMID: 38651406 Free PMC article.
-
Intravenous immunoglobulins (IVIG) in severe/critical COVID-19 adult patients.Biomed Pharmacother. 2023 Jul;163:114851. doi: 10.1016/j.biopha.2023.114851. Epub 2023 May 5. Biomed Pharmacother. 2023. PMID: 37167723 Free PMC article. Review.
-
A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases.Front Immunol. 2023 Mar 27;14:1127339. doi: 10.3389/fimmu.2023.1127339. eCollection 2023. Front Immunol. 2023. PMID: 37051237 Free PMC article. Review.
-
Efficacy and safety of trimodulin in patients with severe COVID-19: results from a randomised, placebo-controlled, double-blind, multicentre, phase II trial (ESsCOVID).Eur J Med Res. 2024 Aug 13;29(1):418. doi: 10.1186/s40001-024-02008-x. Eur J Med Res. 2024. PMID: 39138518 Free PMC article. Clinical Trial.
References
-
- Coronavirus disease (COVID-19) pandemic [https://www.who.int/emergencies/diseases/novel-coronavirus-2019]
-
- Alhazzani W, Moller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, et al. Surviving sepsis campaign: guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous