Customized Design 3D Printed PLGA/Calcium Sulfate Scaffold Enhances Mechanical and Biological Properties for Bone Regeneration
- PMID: 35814012
- PMCID: PMC9260230
- DOI: 10.3389/fbioe.2022.874931
Customized Design 3D Printed PLGA/Calcium Sulfate Scaffold Enhances Mechanical and Biological Properties for Bone Regeneration
Abstract
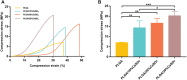
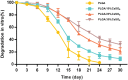
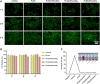
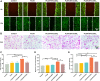
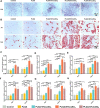
Polylactic glycolic acid copolymer (PLGA) has been widely used in tissue engineering due to its good biocompatibility and degradation properties. However, the mismatched mechanical and unsatisfactory biological properties of PLGA limit further application in bone tissue engineering. Calcium sulfate (CaSO4) is one of the most promising bone repair materials due to its non-immunogenicity, well biocompatibility, and excellent bone conductivity. In this study, aiming at the shortcomings of activity-lack and low mechanical of PLGA in bone tissue engineering, customized-designed 3D porous PLGA/CaSO4 scaffolds were prepared by 3D printing. We first studied the physical properties of PLGA/CaSO4 scaffolds and the results showed that CaSO4 improved the mechanical properties of PLGA scaffolds. In vitro experiments showed that PLGA/CaSO4 scaffold exhibited good biocompatibility. Moreover, the addition of CaSO4 could significantly improve the migration and osteogenic differentiation of MC3T3-E1 cells in the PLGA/CaSO4 scaffolds, and the PLGA/CaSO4 scaffolds made with 20 wt.% CaSO4 exhibited the best osteogenesis properties. Therefore, calcium sulfate was added to PLGA could lead to customized 3D printed scaffolds for enhanced mechanical properties and biological properties. The customized 3D-printed PLGA/CaSO4 scaffold shows great potential for precisely repairing irregular load-bearing bone defects.
Keywords: 3D printing scaffold; biological properties; bone defect; calcium sulfate; mechanical properties; polylactic glycolic acid copolymer.
Copyright © 2022 Liu, Li, Zhao, Chen, Lin, Li, Feng, Jin, Zhang, Wu, Wu, Xu, Ye and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration.Biomater Adv. 2022 Feb;133:112618. doi: 10.1016/j.msec.2021.112618. Epub 2021 Dec 23. Biomater Adv. 2022. PMID: 35031175
-
Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits.Biomaterials. 2018 Jan;153:1-13. doi: 10.1016/j.biomaterials.2017.10.025. Epub 2017 Oct 23. Biomaterials. 2018. PMID: 29096397
-
Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models.Acta Biomater. 2018 Oct 1;79:265-275. doi: 10.1016/j.actbio.2018.08.015. Epub 2018 Aug 18. Acta Biomater. 2018. PMID: 30125670
-
Application of 3D-Printed, PLGA-Based Scaffolds in Bone Tissue Engineering.Int J Mol Sci. 2022 May 23;23(10):5831. doi: 10.3390/ijms23105831. Int J Mol Sci. 2022. PMID: 35628638 Free PMC article. Review.
-
Advances in the Study of Bionic Mineralized Collagen, PLGA, Magnesium Ionomer Materials, and Their Composite Scaffolds for Bone Defect Treatment.J Funct Biomater. 2023 Aug 1;14(8):406. doi: 10.3390/jfb14080406. J Funct Biomater. 2023. PMID: 37623651 Free PMC article. Review.
Cited by
-
Characterization of degradation kinetics of additively manufactured PLGA under variable mechanical loading paradigms.J Mech Behav Biomed Mater. 2024 May;153:106457. doi: 10.1016/j.jmbbm.2024.106457. Epub 2024 Feb 18. J Mech Behav Biomed Mater. 2024. PMID: 38401185
-
The Synergetic Effect of 3D Printing and Electrospinning Techniques in the Fabrication of Bone Scaffolds.Ann Biomed Eng. 2024 Jun;52(6):1518-1533. doi: 10.1007/s10439-024-03500-5. Epub 2024 Mar 26. Ann Biomed Eng. 2024. PMID: 38530536 Review.
-
Enhanced healing of critical-sized bone defects using degradable scaffolds with tailored composition through immunomodulation and angiogenesis.Bioact Mater. 2024 Oct 28;44:371-388. doi: 10.1016/j.bioactmat.2024.10.018. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39539516 Free PMC article.
-
An In Vitro Study of Local Oxygen Therapy as Adjunctive Antimicrobial Therapeutic Option for Patients with Periodontitis.Antibiotics (Basel). 2023 May 31;12(6):990. doi: 10.3390/antibiotics12060990. Antibiotics (Basel). 2023. PMID: 37370309 Free PMC article.
-
Beyond hype: unveiling the Real challenges in clinical translation of 3D printed bone scaffolds and the fresh prospects of bioprinted organoids.J Nanobiotechnology. 2024 Aug 21;22(1):500. doi: 10.1186/s12951-024-02759-z. J Nanobiotechnology. 2024. PMID: 39169401 Free PMC article. Review.
References
-
- Arun Kumar R., Sivashanmugam A., Deepthi S., Bumgardner J. D., Nair S. V., Jayakumar R. (2016). Nano-fibrin Stabilized CaSO 4 Crystals Incorporated Injectable Chitin Composite Hydrogel for Enhanced Angiogenesis & Osteogenesis. Carbohydr. Polym. 140, 144–153. 10.1016/j.carbpol.2015.11.074 - DOI - PubMed
LinkOut - more resources
Full Text Sources

