Nanostructured Lipid Carrier-Based Delivery of Pioglitazone for Treatment of Type 2 Diabetes
- PMID: 35903327
- PMCID: PMC9315350
- DOI: 10.3389/fphar.2022.934156
Nanostructured Lipid Carrier-Based Delivery of Pioglitazone for Treatment of Type 2 Diabetes
Abstract
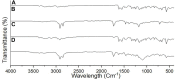
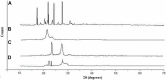
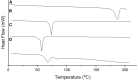
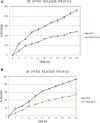
Pioglitazone (PGZ) is utilized as a therapeutic agent in the management of (type 2) diabetes to control blood glucose levels. The existing research work was intended to make and optimize PGZ-containing NLCs (nanostructured lipid carriers). The fabricated nanostructured lipid carrier preparation was optimized by using different concentrations of the surfactants (Tween 80 and Span 80) and solid lipid (Compritol® 888 ATO) and liquid lipid (Labrasol®) while keeping the concentration of drug (PGZ), and co-surfactants (poloxamer 188) the same. The optimized NLC formulation (PGZ-NLCs) was further assessed for physical and chemical characterization, in vitro PGZ release, and stability studies. The optimized PGZ-NLCs have shown an average diameter of 150.4 nm, EE of 92.53%, PDI value of 0.076, and zeta-potential of -29.1 mV, correspondingly. The DSC thermal analysis and XRD diffractograms had not presented the spectrum of PGZ, confirming the comprehensive encapsulation of PGZ in the lipid core. PGZ-NLCs showed significantly extended release (51% in 24 h) compared to the unformulated PGZ. Our study findings confirmed that PGZ-NLCs can be a promising drug delivery system for the treatment of type 2 diabetes.
Keywords: NLCs; diabetes; nanoparticles; pioglitazone; poor aqueous solubility.
Copyright © 2022 Ilyas, Asif, Wang, Altaf, Zafar, Faran Ashraf Baig, Paiva-Santos and Abbas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Nanostructured lipid carriers as a strategy for encapsulation of active plant constituents: Formulation and in vitro physicochemical characterizations.Chem Phys Lipids. 2021 Mar;235:105037. doi: 10.1016/j.chemphyslip.2020.105037. Epub 2021 Jan 2. Chem Phys Lipids. 2021. PMID: 33400968
-
Formulation, Characterization, and Pharmacokinetic Studies of 6-Gingerol-Loaded Nanostructured Lipid Carriers.AAPS PharmSciTech. 2018 Nov;19(8):3661-3669. doi: 10.1208/s12249-018-1165-2. Epub 2018 Oct 15. AAPS PharmSciTech. 2018. PMID: 30324361
-
Systematic implementation of quality-by-design (QbD) to develop NSAID-loaded nanostructured lipid carriers for ocular application: preformulation screening studies and statistical hybrid-design for optimization of variables.Drug Dev Ind Pharm. 2020 Mar;46(3):443-455. doi: 10.1080/03639045.2020.1724135. Epub 2020 Feb 9. Drug Dev Ind Pharm. 2020. PMID: 32037896
-
Leveraging nanostructured lipid carriers to enhance _targeted delivery and efficacy in breast cancer therapy: a comprehensive review.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug 28. doi: 10.1007/s00210-024-03408-w. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39196394 Review.
-
Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies.Saudi Pharm J. 2021 Sep;29(9):999-1012. doi: 10.1016/j.jsps.2021.07.015. Epub 2021 Jul 21. Saudi Pharm J. 2021. PMID: 34588846 Free PMC article. Review.
Cited by
-
Investigation of anti-diabetic potential and molecular simulation studies of dihydropyrimidinone derivatives.Front Endocrinol (Lausanne). 2022 Oct 12;13:1022623. doi: 10.3389/fendo.2022.1022623. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36313779 Free PMC article.
-
Discovery of 2-deoxy glucose surfaced mixed layer dendrimer: a smart neuron _targeted systemic drug delivery system for brain diseases.Theranostics. 2024 May 19;14(8):3221-3245. doi: 10.7150/thno.95476. eCollection 2024. Theranostics. 2024. PMID: 38855177 Free PMC article.
-
Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes.Sci Rep. 2024 Jan 5;14(1):638. doi: 10.1038/s41598-024-51210-8. Sci Rep. 2024. PMID: 38182717 Free PMC article.
References
-
- AmeeduzzafarEl-Bagory I., El-Bagory I., Alruwaili N. K., Elkomy M. H., Ahmad J., Afzal M., et al. (2019). Development of Novel Dapagliflozin Loaded Solid Self-Nanoemulsifying Oral Delivery System: Physiochemical Characterization and In Vivo Antidiabetic Activity. J. Drug Deliv. Sci. Technol. 54, 101279. 10.1016/j.jddst.2019.101279 - DOI
-
- Bhosale U., Galgatte U., Chaudhari P. (2016). Development of Pioglitazone Hydrochloride Lipospheres by Melt Dispersion Technique: Optimization and Evaluation. J. App Pharm. Sci. 6 (01), 107–117. 10.7324/japs.2016.600118 - DOI
-
- Eckland D., Danhof M. (2000). Clinical Pharmacokinetics of Pioglitazone. Exp. Clin. Endocrinol. diabetes 108 (Suppl. 2), 234–242. 10.1055/s-2000-8525 - DOI
-
- Elbary A. A., Kassem M. A., Abou Samra M. M., Khalil R. M. (2008). Formulation and Hypoglycemic Activity of Pioglitazone-Cyclodextrin Inclusion Complexes. Drug Discov. Ther. 2 (2), 94–107. - PubMed
LinkOut - more resources
Full Text Sources

